C-Nucleophiles
For reactivities of structurally related compounds go to: Nucleophiles
Found 572 Molecules.
| Molecule | Solvent | Reactivity Parameters | Classification | Reference (sort by title or year) |
|---|---|---|---|---|
 Z-3-(trimethylsiloxy)pent-2-ene | dichloromethane | N Parameter: 5.58 sN Parameter: 1.00 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
 trimethyl(prenyl)silane | dichloromethane | N Parameter: 0.90 sN Parameter: 1.17 | J. Am. Chem. Soc. 2001, 123, 9500-9512 DOI: 10.1021/ja010890y | |
 toluene | dichloromethane | N Parameter: -4.36 sN Parameter: 1.77 | J. Am. Chem. Soc. 2012, 134, 13902-13911 DOI: 10.1021/ja306522b | |
 thiophene | dichloromethane | N Parameter: -1.01 sN Parameter: 1.10 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
 tetramethylethylene | dichloromethane | N Parameter: -1.00 sN Parameter: 1.40 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
 tetrakis(5-methyl-furan-2-yl)borate | MeCN | N Parameter: 9.09 sN Parameter: 1.12 | Chem. Sci. 2012, 3, 878-882 DOI: 10.1039/c2sc00883a | |
 tert-butyl isocyanide | dichloromethane | N Parameter: 5.47 sN Parameter: 0.77 | Angew. Chem. Int. Ed. 2007, 46, 3563-3566 DOI: 10.1002/anie.200605205 | |
 tert-butyl indole-1-carboxylate | MeCN | N Parameter: 1.68 sN Parameter: 1.26 | J. Am. Chem. Soc. 2013, 135, 6317-6324 DOI: 10.1021/ja4017655 | |
 styrene | dichloromethane | N Parameter: 0.78 sN Parameter: 0.95 | J. Am. Chem. Soc. 2001, 123, 9500-9512 DOI: 10.1021/ja010890y | |
 sodium indenide (in DMSO) | DMSO | N Parameter: 23.74 sN Parameter: 0.71 | Angew. Chem. Int. Ed. 2015, 54, 12497-12500 DOI: 10.1002/anie.201501385 | |
 pyrrole | dichloromethane | N Parameter: 4.63 sN Parameter: 1.00 | Chem. Eur. J. 2003, 9, 2209-2218 DOI: 10.1002/chem.200204666 | |
 propene | dichloromethane | N Parameter: -2.41 sN Parameter: 1.00 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
 prenyl-tributylstannane | dichloromethane | N Parameter: 4.74 sN Parameter: 1.15 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
 potassium trifluoro(furan-3-yl)borate | MeCN | N Parameter: 6.83 sN Parameter: 0.93 | J. Am. Chem. Soc. 2013, 135, 6317-6324 DOI: 10.1021/ja4017655 | |
 potassium trifluoro(3-methoxy-thiophen-2-yl)borate | MeCN | N Parameter: 7.32 sN Parameter: 0.90 | J. Am. Chem. Soc. 2013, 135, 6317-6324 DOI: 10.1021/ja4017655 | |
 potassium trifluoro(1-methyl-1H-indol-5-yl)borate | MeCN | N Parameter: 8.77 sN Parameter: 1.09 | J. Am. Chem. Soc. 2013, 135, 6317-6324 DOI: 10.1021/ja4017655 | |
 potassium trifluoro(1-methyl-1H-indol-2-yl)borate | MeCN | N Parameter: 9.55 sN Parameter: 1.16 | J. Am. Chem. Soc. 2013, 135, 6317-6324 DOI: 10.1021/ja4017655 | |
 potassium indenide (in DMSO) | DMSO | N Parameter: 24.16 sN Parameter: 0.68 | Angew. Chem. Int. Ed. 2015, 54, 12497-12500 DOI: 10.1002/anie.201501385 | |
 potassium allyltrifluoroborate (in MeCN) | MeCN | N Parameter: 5.29 sN Parameter: 0.87 | J. Am. Chem. Soc. 2017, 139, 15324-15327 DOI: 10.1021/jacs.7b10240 | |
 potassium (1-(tert-butoxycarbonyl)-5-methoxy-1H-indol-2-yl)trifluoroborate | MeCN | N Parameter: 7.10 sN Parameter: 1.18 | J. Am. Chem. Soc. 2013, 135, 6317-6324 DOI: 10.1021/ja4017655 | |
 potassium (1-(tert-butoxycarbonyl)-1H-indol-3-yl)trifluoroborate | MeCN | N Parameter: 4.06 sN Parameter: 0.79 | J. Am. Chem. Soc. 2013, 135, 6317-6324 DOI: 10.1021/ja4017655 | |
 potassium (1-(tert-butoxycarbonyl)-1H-indol-2-yl)trifluoroborate | MeCN | N Parameter: 6.46 sN Parameter: 0.96 | J. Am. Chem. Soc. 2013, 135, 6317-6324 DOI: 10.1021/ja4017655 | |
 phenyldiazomethane | dichloromethane | N Parameter: 9.35 sN Parameter: 0.83 | Chem. Eur. J. 2003, 9, 4068-4076 DOI: 10.1002/chem.200304913 | |
 phenylacetylene | dichloromethane | N Parameter: -0.04 sN Parameter: 0.77 | Angew. Chem. Int. Ed. 2014, 53, 4968-4971 DOI: 10.1002/anie.201402055 | |
 Ph3P=CH-COPh | dichloromethane | N Parameter: 9.54 sN Parameter: 0.97 | J. Am. Chem. Soc. 2009, 131, 704-714 DOI: 10.1021/ja8056216 | |
 Ph3P=CH-CO2Et (in DMSO) | DMSO | N Parameter: 12.21 sN Parameter: 0.62 | J. Am. Chem. Soc. 2009, 131, 704-714 DOI: 10.1021/ja8056216 | |
 Ph3P=CH-CO2Et | dichloromethane | N Parameter: 12.79 sN Parameter: 0.77 | J. Am. Chem. Soc. 2009, 131, 704-714 DOI: 10.1021/ja8056216 | |
 Ph3P=CH-CN | dichloromethane | N Parameter: 12.29 sN Parameter: 0.75 | J. Am. Chem. Soc. 2009, 131, 704-714 DOI: 10.1021/ja8056216 | |
 Ph3P=CH-CHO (in MeCN) | MeCN | N Parameter: 9.09 sN Parameter: 0.74 | J. Am. Chem. Soc. 2016, 138, 11272-11281 DOI: 10.1021/jacs.6b06264 | |
 Ph3P=CH-C(O)Me (in MeCN) | MeCN | N Parameter: 10.27 sN Parameter: 0.83 | J. Am. Chem. Soc. 2016, 138, 11272-11281 DOI: 10.1021/jacs.6b06264 | |
 Ph3P=C(CH3)-CO2Et | dichloromethane | N Parameter: 13.09 sN Parameter: 0.73 | J. Am. Chem. Soc. 2009, 131, 704-714 DOI: 10.1021/ja8056216 | |
 Ph2P(O)CH(-)CO2Et (in DMSO) | DMSO | N Parameter: 19.20 sN Parameter: 0.69 | J. Am. Chem. Soc. 2009, 131, 704-714 DOI: 10.1021/ja8056216 | |
 Ph2P(O)CH(-)CN (in DMSO) | DMSO | N Parameter: 18.69 sN Parameter: 0.72 | J. Am. Chem. Soc. 2009, 131, 704-714 DOI: 10.1021/ja8056216 | |
 p-tosylmethyl isocyanide (TosMIC) | dichloromethane | N Parameter: 3.50 sN Parameter: 0.76 | Angew. Chem. Int. Ed. 2007, 46, 3563-3566 DOI: 10.1002/anie.200605205 | |
 norbornene | dichloromethane | N Parameter: -0.25 sN Parameter: 1.09 | J. Am. Chem. Soc. 2012, 134, 13902-13911 DOI: 10.1021/ja306522b | |
 N-vinylcarbazole | dichloromethane | N Parameter: 5.02 sN Parameter: 0.94 | Macromolecules 2002, 35, 5454-5458 DOI: 10.1021/ma020306l | |
 N-methylpyrrole | dichloromethane | N Parameter: 5.85 sN Parameter: 1.03 | J. Am. Chem. Soc. 2001, 123, 9500-9512 DOI: 10.1021/ja010890y | |
 N-methylindole | dichloromethane | N Parameter: 5.75 sN Parameter: 1.23 | J. Org. Chem. 2006, 71, 9088-9095 DOI: 10.1021/jo0614339 | |
 N-methylenepyrrolidin-1-amine (in CH2Cl2) | dichloromethane | N Parameter: 7.84 sN Parameter: 0.89 | Angew. Chem. Int. Ed. 2013, 52, 11900-11904 DOI: 10.1002/anie.201305092 | |
 n-butyl vinyl ether | dichloromethane | N Parameter: 3.76 sN Parameter: 0.91 | J. Am. Chem. Soc. 2012, 134, 13902-13911 DOI: 10.1021/ja306522b | |
 N-benzyl-N-(but-1-yn-1-yl)-4-methylbenzenesulfonamide | dichloromethane | N Parameter: 5.16 sN Parameter: 0.85 | Angew. Chem. Int. Ed. 2014, 53, 4968-4971 DOI: 10.1002/anie.201402055 | |
 N-benzyl-N-((2-methoxyphenyl)ethynyl)-4-methylbenzenesulfonamide | dichloromethane | N Parameter: 4.40 sN Parameter: 0.86 | Angew. Chem. Int. Ed. 2014, 53, 4968-4971 DOI: 10.1002/anie.201402055 | |
 N-(triisopropylsilyl)pyrrole | dichloromethane | N Parameter: 3.12 sN Parameter: 0.93 | Chem. Eur. J. 2003, 9, 2209-2218 DOI: 10.1002/chem.200204666 | |
 N-(cyclopent-1-en-1-yl)acetamide | MeCN | N Parameter: 7.06 sN Parameter: 0.87 | Chem. Eur. J. 2012, 18, 5732-5740 DOI: 10.1002/chem.201103519 | |
 N-(cyclohex-1-en-1-yl)acetamide | MeCN | N Parameter: 5.64 sN Parameter: 0.79 | Chem. Eur. J. 2012, 18, 5732-5740 DOI: 10.1002/chem.201103519 | |
 N-(3,4-dihydronaphthalen-1-yl)acetamide | MeCN | N Parameter: 4.91 sN Parameter: 0.87 | Chem. Eur. J. 2012, 18, 5732-5740 DOI: 10.1002/chem.201103519 | |
 N-(3,3-dimethylbut-1-en-2-yl)acetamide | MeCN | N Parameter: 4.61 sN Parameter: 0.98 | Chem. Eur. J. 2012, 18, 5732-5740 DOI: 10.1002/chem.201103519 | |
 N-(1-phenylvinyl)benzamide | MeCN | N Parameter: 5.44 sN Parameter: 1.00 | Chem. Eur. J. 2012, 18, 5732-5740 DOI: 10.1002/chem.201103519 | |
 N-(1-phenylvinyl)acetamide | MeCN | N Parameter: 5.73 sN Parameter: 0.97 | Chem. Eur. J. 2012, 18, 5732-5740 DOI: 10.1002/chem.201103519 | |
 N-(1-(p-tolyl)vinyl)acetamide | MeCN | N Parameter: 6.57 sN Parameter: 0.91 | Chem. Eur. J. 2012, 18, 5732-5740 DOI: 10.1002/chem.201103519 | |
 N-(1-(naphthalen-2-yl)vinyl)acetamide | MeCN | N Parameter: 6.28 sN Parameter: 0.95 | Chem. Eur. J. 2012, 18, 5732-5740 DOI: 10.1002/chem.201103519 | |
 N-(1-(4-methoxyphenyl)vinyl)acetamide | MeCN | N Parameter: 7.06 sN Parameter: 0.85 | Chem. Eur. J. 2012, 18, 5732-5740 DOI: 10.1002/chem.201103519 | |
 N-(1-(4-chlorophenyl)vinyl)acetamide | MeCN | N Parameter: 5.60 sN Parameter: 1.00 | Chem. Eur. J. 2012, 18, 5732-5740 DOI: 10.1002/chem.201103519 | |
 N,N-Dimethylbarbituric acid iodonium ylide (in CHCl2) | dichloromethane | N Parameter: 7.98 sN Parameter: 0.71 | J. Am. Chem. Soc. 2016, 138, 10304-10313 DOI: 10.1021/jacs.6b05768 | |
 morpholinoisobutylene | dichloromethane | N Parameter: 10.04 sN Parameter: 0.82 | Chem. Eur. J. 2003, 9, 2209-2218 DOI: 10.1002/chem.200204666 | |
 methylenecycloundecane | dichloromethane | N Parameter: 2.33 sN Parameter: 0.90 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
 methylenecyclopropane | dichloromethane | N Parameter: -0.47 sN Parameter: 0.90 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
 methylenecyclopentane | dichloromethane | N Parameter: 2.82 sN Parameter: 0.90 | J. Am. Chem. Soc. 2001, 123, 9500-9512 DOI: 10.1021/ja010890y | |
 methylenecyclopentadecane | dichloromethane | N Parameter: 1.69 sN Parameter: 0.90 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
 methylenecyclooctane | dichloromethane | N Parameter: 3.16 sN Parameter: 0.90 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
 methylenecyclononane | dichloromethane | N Parameter: 2.57 sN Parameter: 0.90 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
 methylenecyclohexane | dichloromethane | N Parameter: 1.16 sN Parameter: 1.04 | J. Am. Chem. Soc. 2012, 134, 13902-13911 DOI: 10.1021/ja306522b | |
 methylenecycloheptane | dichloromethane | N Parameter: 2.24 sN Parameter: 0.90 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
 methylenecyclododecane | dichloromethane | N Parameter: 1.52 sN Parameter: 0.90 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
 methylenecyclodecane | dichloromethane | N Parameter: 2.82 sN Parameter: 0.90 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
 methylenecyclobutane | dichloromethane | N Parameter: 1.65 sN Parameter: 0.90 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
 methyl diazoacetate | dichloromethane | N Parameter: 4.68 sN Parameter: 0.94 | J. Am. Chem. Soc. 2023, 145, 7416-7434 DOI: 10.1021/jacs.2c13872 | |
 methyl (cyclohexen-1-yl)prolinate (in MeCN) | MeCN | N Parameter: 14.96 sN Parameter: 0.68 | Angew. Chem. Int. Ed. 2010, 49, 9526-9529 DOI: 10.1002/anie.201004344 | |
 methoxybis(phenylsulfonyl)methanide (in DMSO) | DMSO | N Parameter: 17.29 sN Parameter: 0.70 | Angew. Chem. Int. Ed. 2016, 55, 12845-12848 DOI: 10.1002/anie.201605616 | |
 MeSO2-CH-CO2Et (in DMSO) | DMSO | N Parameter: 18.00 sN Parameter: 0.66 | Chem. Eur. J. 2010, 16, 8610-8614 DOI: 10.1002/chem.201001455 | |
 MeSO-CH-CO2Et (in DMSO) | DMSO | N Parameter: 20.61 sN Parameter: 0.64 | Chem. Eur. J. 2010, 16, 8610-8614 DOI: 10.1002/chem.201001455 | |
 MeO-Breslow 1e | THF | N Parameter: 15.65 sN Parameter: 0.52 | Angew. Chem. Int. Ed. 2012, 51, 10408-10412 DOI: 10.1002/anie.201204524 | |
 MeO-Breslow 1c | THF | N Parameter: 16.61 sN Parameter: 0.68 | Angew. Chem. Int. Ed. 2012, 51, 10408-10412 DOI: 10.1002/anie.201204524 | |
 MeO-Breslow 1b | THF | N Parameter: 10.45 sN Parameter: 0.81 | Angew. Chem. Int. Ed. 2012, 51, 10408-10412 DOI: 10.1002/anie.201204524 | |
 MeO-Breslow 1a | THF | N Parameter: 14.77 sN Parameter: 0.80 | Angew. Chem. Int. Ed. 2012, 51, 10408-10412 DOI: 10.1002/anie.201204524 | |
 Meldrum's acid iodonium ylide (in CH2Cl2) | dichloromethane | N Parameter: 4.36 sN Parameter: 1.06 | J. Am. Chem. Soc. 2016, 138, 10304-10313 DOI: 10.1021/jacs.6b05768 | |
 Me2S=CH-CO2Et (in DMSO) | DMSO | N Parameter: 15.85 sN Parameter: 0.61 | Chem. Eur. J. 2010, 16, 8610-8614 DOI: 10.1002/chem.201001455 | |
 Me2S=CH-CN (in DMSO) | DMSO | N Parameter: 16.23 sN Parameter: 0.60 | Chem. Eur. J. 2010, 16, 8610-8614 DOI: 10.1002/chem.201001455 | |
 Me2S=CH(p-NO2-C6H4) (in DMSO) | DMSO | N Parameter: 18.42 sN Parameter: 0.65 | J. Am. Chem. Soc. 2010, 132, 17894-17900 DOI: 10.1021/ja1084749 | |
 Me2S=CH(4-CN-C6H4) (in DMSO) | DMSO | N Parameter: 21.07 sN Parameter: 0.68 | J. Am. Chem. Soc. 2010, 132, 17894-17900 DOI: 10.1021/ja1084749 | |
 Me2S(O)=CH2 (in DMSO) | DMSO | N Parameter: 21.29 sN Parameter: 0.47 | J. Am. Chem. Soc. 2010, 132, 17894-17900 DOI: 10.1021/ja1084749 | |
 m-xylene | dichloromethane | N Parameter: -3.57 sN Parameter: 2.08 | J. Am. Chem. Soc. 2001, 123, 9500-9512 DOI: 10.1021/ja010890y | |
 lithium indenide (in DMSO) | DMSO | N Parameter: 23.66 sN Parameter: 0.70 | Angew. Chem. Int. Ed. 2015, 54, 12497-12500 DOI: 10.1002/anie.201501385 | |
 lithium bis(5-methylthiophen-2-yl)pinacolborate | MeCN | N Parameter: 7.67 sN Parameter: 0.87 | Angew. Chem. Int. Ed. 2015, 54, 2780-2783 DOI: 10.1002/anie.201410562 | |
 lithium 5,5-dimethyl-2-(1-phenylethyl)-2-(4-(trifluoromethyl)phenyl)-1,3,2-dioxaborinan-2-uide (in MeCN) | MeCN | N Parameter: 11.29 sN Parameter: 0.76 | Org. Lett. 2015, 17, 2614-2617 DOI: 10.1021/acs.orglett.5b00918 | |
 lithium 4,4,5,5-tetramethyl-2-phenyl-2-(prop-1-en-2-yl)-1,3,2-dioxaborolan-2-uide (in MeCN) | MeCN | N Parameter: 11.00 sN Parameter: 0.57 | J. Am. Chem. Soc. 2022, 144, 16118-16130 DOI: 10.1021/jacs.2c06493 | |
 lithium 4,4,5,5-tetramethyl-2-phenyl-2-(1-phenylethyl)-1,3,2-dioxaborolan-2-uide (in MeCN) | MeCN | N Parameter: 10.97 sN Parameter: 0.63 | Org. Lett. 2015, 17, 2614-2617 DOI: 10.1021/acs.orglett.5b00918 | |
 lithium 4,4,5,5-tetramethyl-2-(1-phenylethyl)-2-(4-(trifluoromethyl)phenyl)-1,3,2-dioxaborolan-2-uide (in MeCN) | MeCN | N Parameter: 8.56 sN Parameter: 0.76 | Org. Lett. 2015, 17, 2614-2617 DOI: 10.1021/acs.orglett.5b00918 | |
 lithium 2-isopropyl-4,4,5,5-tetramethyl-2-(4-(trifluoromethyl)phenyl)-1,3,2-dioxaborolan-2-uide (in MeCN) | MeCN | N Parameter: 6.63 sN Parameter: 0.75 | Org. Lett. 2015, 17, 2614-2617 DOI: 10.1021/acs.orglett.5b00918 | |
 lithium 2-ethyl-4,4,5,5-tetramethyl-2-(4-(trifluoromethyl)phenyl)-1,3,2-dioxaborolan-2-uide (in MeCN) | MeCN | N Parameter: 7.68 sN Parameter: 0.78 | Org. Lett. 2015, 17, 2614-2617 DOI: 10.1021/acs.orglett.5b00918 | |
 lithium 2-cyclopropyl-4,4,5,5-tetramethyl-2-(4-(trifluoromethyl)phenyl)-1,3,2-dioxaborolan-2-uide (in MeCN) | MeCN | N Parameter: 7.72 sN Parameter: 0.75 | Org. Lett. 2015, 17, 2614-2617 DOI: 10.1021/acs.orglett.5b00918 | |
 lithium 2-benzyl-4,4,5,5-tetramethyl-2-phenyl-1,3,2-dioxaborolan-2-uide (in MeCN) | MeCN | N Parameter: 8.92 sN Parameter: 0.70 | Org. Lett. 2015, 17, 2614-2617 DOI: 10.1021/acs.orglett.5b00918 | |
 lithium 2-benzyl-4,4,5,5-tetramethyl-2-(4-(trifluoromethyl)phenyl)-1,3,2-dioxaborolan-2-uide (in MeCN) | MeCN | N Parameter: 7.15 sN Parameter: 0.77 | Org. Lett. 2015, 17, 2614-2617 DOI: 10.1021/acs.orglett.5b00918 | |
 lithium 2-benzyl-2-(3,5-bis(trifluoromethyl)phenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-uide (in MeCN) | MeCN | N Parameter: 6.30 sN Parameter: 0.78 | Org. Lett. 2015, 17, 2614-2617 DOI: 10.1021/acs.orglett.5b00918 | |
 lithium 2-allyl-4,4,5,5-tetramethyl-2-phenyl-1,3,2-dioxaborolan-2-uide (in MeCN) | MeCN | N Parameter: 11.20 sN Parameter: 0.64 | J. Am. Chem. Soc. 2017, 139, 15324-15327 DOI: 10.1021/jacs.7b10240 | |
 lithium 2-allyl-2-(3,5-bis(trifluoromethyl)phenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-uide (in MeCN) | MeCN | N Parameter: 8.71 sN Parameter: 0.80 | J. Am. Chem. Soc. 2017, 139, 15324-15327 DOI: 10.1021/jacs.7b10240 | |
 lithium 2-(3,5-bis(trifluoromethyl)phenyl)-4,4,5,5-tetramethyl-2-(1-phenylethyl)-1,3,2-dioxaborolan-2-uide (in MeCN) | MeCN | N Parameter: 7.46 sN Parameter: 0.76 | Org. Lett. 2015, 17, 2614-2617 DOI: 10.1021/acs.orglett.5b00918 | |
 lithium (E)-2-(3,5-bis(trifluoromethyl)phenyl)-2-(but-2-en-1-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-uide (in MeCN) | MeCN | N Parameter: 9.49 sN Parameter: 0.82 | J. Am. Chem. Soc. 2017, 139, 15324-15327 DOI: 10.1021/jacs.7b10240 | |
 lithium (5-methylthiophen-2-yl)(phenyl)pinacolborate | MeCN | N Parameter: 7.24 sN Parameter: 0.83 | Angew. Chem. Int. Ed. 2015, 54, 2780-2783 DOI: 10.1002/anie.201410562 | |
 lithium (5-methylthiophen-2-yl)(4-methylphenyl)pinacolborate | MeCN | N Parameter: 6.98 sN Parameter: 0.93 | Angew. Chem. Int. Ed. 2015, 54, 2780-2783 DOI: 10.1002/anie.201410562 | |
 lithium (5-methylthiophen-2-yl)(4-methoxyphenyl)pinacolborate | MeCN | N Parameter: 7.51 sN Parameter: 0.87 | Angew. Chem. Int. Ed. 2015, 54, 2780-2783 DOI: 10.1002/anie.201410562 | |
 lithium (5-methylthiophen-2-yl)(4-chlorophenyl)pinacolborate | MeCN | N Parameter: 6.77 sN Parameter: 0.88 | Angew. Chem. Int. Ed. 2015, 54, 2780-2783 DOI: 10.1002/anie.201410562 | |
 lithium (5-methylthiophen-2-yl)(4-(trifluoromethyl)phenyl)neopentylglycolborate | MeCN | N Parameter: 11.85 sN Parameter: 0.72 | Angew. Chem. Int. Ed. 2015, 54, 2780-2783 DOI: 10.1002/anie.201410562 | |
 lithium (5-methylthiophen-2-yl)(4-(trifluoromethyl)phenyl)catecholglycolborate | MeCN | N Parameter: 6.50 sN Parameter: 0.77 | Angew. Chem. Int. Ed. 2015, 54, 2780-2783 DOI: 10.1002/anie.201410562 | |
 lithium (5-methylthiophen-2-yl)(4-(dimethylamino)phenyl)pinacolborate | MeCN | N Parameter: 8.02 sN Parameter: 0.89 | Angew. Chem. Int. Ed. 2015, 54, 2780-2783 DOI: 10.1002/anie.201410562 | |
 lithium (5-methylthiophen-2-yl)(3,5-bis(trifluoromethyl)phenyl)pinacolborate | MeCN | N Parameter: 5.53 sN Parameter: 1.00 | Angew. Chem. Int. Ed. 2015, 54, 2780-2783 DOI: 10.1002/anie.201410562 | |
 lithium (5-methylthiophen-2-yl)(3,5-bis(trifluoromethyl)phenyl)neopentylglycolborate | MeCN | N Parameter: 10.13 sN Parameter: 0.91 | Angew. Chem. Int. Ed. 2015, 54, 2780-2783 DOI: 10.1002/anie.201410562 | |
 lithium (5-methylthiophen-2-yl)(3,5-bis(trifluoromethyl)phenyl)glycolborate | MeCN | N Parameter: 11.23 sN Parameter: 0.77 | Angew. Chem. Int. Ed. 2015, 54, 2780-2783 DOI: 10.1002/anie.201410562 | |
 lithium (5-methylfuran-2-yl)(4-(trifluoromethyl)phenyl)pinacolborate | MeCN | N Parameter: 8.13 sN Parameter: 0.85 | Angew. Chem. Int. Ed. 2015, 54, 2780-2783 DOI: 10.1002/anie.201410562 | |
 isobutylenyl-ethylether | dichloromethane | N Parameter: 4.23 sN Parameter: 1.00 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
 Indole | dichloromethane | N Parameter: 5.55 sN Parameter: 1.09 | J. Org. Chem. 2006, 71, 9088-9095 DOI: 10.1021/jo0614339 | |
 furan | dichloromethane | N Parameter: 1.33 sN Parameter: 1.29 | J. Am. Chem. Soc. 2012, 134, 13902-13911 DOI: 10.1021/ja306522b | |
 fluorobis(phenylsulfonyl)methanide (in DMSO) | DMSO | N Parameter: 17.46 sN Parameter: 0.73 | Angew. Chem. Int. Ed. 2016, 55, 12845-12848 DOI: 10.1002/anie.201605616 | |
 ethyl-vinylether | dichloromethane | N Parameter: 3.92 sN Parameter: 0.90 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
 ethyl diazoacetate | dichloromethane | N Parameter: 4.91 sN Parameter: 0.95 | Chem. Eur. J. 2003, 9, 4068-4076 DOI: 10.1002/chem.200304913 | |
 ethyl (E)-3-(N-morpholino)acrylate | dichloromethane | N Parameter: 8.52 sN Parameter: 0.80 | Chem. Eur. J. 2003, 9, 2209-2218 DOI: 10.1002/chem.200204666 | |
 ethyl (E)-3-(dimethylamino)acrylate | dichloromethane | N Parameter: 9.43 sN Parameter: 0.80 | Chem. Eur. J. 2003, 9, 2209-2218 DOI: 10.1002/chem.200204666 | |
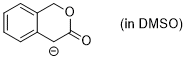 enolate of 3-isochromanone (in DMSO) | DMSO | N Parameter: 25.39 sN Parameter: 0.54 | J. Org. Chem. 2024, 89, 6915-6928 DOI: 10.1021/acs.joc.4c00277 | |
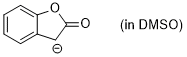 enolate of 2-coumaranone (in DMSO) | DMSO | N Parameter: 19.60 sN Parameter: 0.75 | J. Org. Chem. 2024, 89, 6915-6928 DOI: 10.1021/acs.joc.4c00277 | |
 diphenyldiazomethane | dichloromethane | N Parameter: 5.29 sN Parameter: 0.92 | Chem. Eur. J. 2003, 9, 4068-4076 DOI: 10.1002/chem.200304913 | |
 dimethyl diazomalonate | dichloromethane | N Parameter: -1.24 sN Parameter: 0.81 | J. Am. Chem. Soc. 2023, 145, 7416-7434 DOI: 10.1021/jacs.2c13872 | |
 Dimethyl bis(indenyl)zirconium(IV) | dichloromethane | N Parameter: 6.89 sN Parameter: 1.00 | Chem. Eur. J. 2016, 22, 11196-11200 DOI: 10.1002/chem.201602452 | |
 Dimethyl 1,1'-isopropylidenezirconocene | dichloromethane | N Parameter: 5.20 sN Parameter: 1.00 | Chem. Eur. J. 2016, 22, 11196-11200 DOI: 10.1002/chem.201602452 | |
 Dimedone iodonium ylide (in CH2Cl2) | dichloromethane | N Parameter: 6.18 sN Parameter: 0.81 | J. Am. Chem. Soc. 2016, 138, 10304-10313 DOI: 10.1021/jacs.6b05768 | |
 diethyl diazomalonate | dichloromethane | N Parameter: -0.35 sN Parameter: 0.93 | Chem. Eur. J. 2003, 9, 4068-4076 DOI: 10.1002/chem.200304913 | |
 diazomethane | dichloromethane | N Parameter: 10.48 sN Parameter: 0.78 | Chem. Eur. J. 2003, 9, 4068-4076 DOI: 10.1002/chem.200304913 | |
 diazocyclopentadiene (in CH2Cl2) | dichloromethane | N Parameter: 4.84 sN Parameter: 1.06 | Synthesis 2023, 55, 354-358 DOI: 10.1055/s-0041-1737327 | |
 diazoacetone | dichloromethane | N Parameter: 3.96 sN Parameter: 0.91 | Chem. Eur. J. 2003, 9, 4068-4076 DOI: 10.1002/chem.200304913 | |
 Dexoxy Breslow intermediate 6 | THF | N Parameter: 15.58 sN Parameter: 0.57 | Angew. Chem. Int. Ed. 2012, 51, 10408-10412 DOI: 10.1002/anie.201204524 | |
 Desoxy Breslow intermediate 2c' | THF | N Parameter: 12.75 sN Parameter: 0.71 | Angew. Chem. Int. Ed. 2012, 51, 6231-6235 DOI: 10.1002/anie.201202327 | |
 Desoxy Breslow intermediate 2b' | THF | N Parameter: 11.42 sN Parameter: 0.70 | Angew. Chem. Int. Ed. 2012, 51, 6231-6235 DOI: 10.1002/anie.201202327 | |
 Desoxy Breslow intermediate 2b | THF | N Parameter: 13.91 sN Parameter: 0.64 | Angew. Chem. Int. Ed. 2012, 51, 6231-6235 DOI: 10.1002/anie.201202327 | |
 Desoxy Breslow intermediate 2a' | THF | N Parameter: 14.45 sN Parameter: 0.71 | Angew. Chem. Int. Ed. 2012, 51, 6231-6235 DOI: 10.1002/anie.201202327 | |
 Desoxy Breslow intermediate 2a | THF | N Parameter: 17.12 sN Parameter: 0.80 | Angew. Chem. Int. Ed. 2012, 51, 6231-6235 DOI: 10.1002/anie.201202327 | |
 Desoxy Breslow intermediate 2a | DMSO | N Parameter: 17.41 sN Parameter: 0.74 | Angew. Chem. Int. Ed. 2012, 51, 6231-6235 DOI: 10.1002/anie.201202327 | |
 Danishefskys diene | dichloromethane | N Parameter: 8.57 sN Parameter: 0.84 | J. Am. Chem. Soc. 2001, 123, 9500-9512 DOI: 10.1021/ja010890y | |
 cyclopentene | dichloromethane | N Parameter: -1.55 sN Parameter: 1.10 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
 cyclopentadiene | dichloromethane | N Parameter: 2.30 sN Parameter: 1.06 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
 cycloocta-1,3-diene | dichloromethane | N Parameter: -0.50 sN Parameter: 1.10 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
 cyclohexa-1,3-diene | dichloromethane | N Parameter: 0.67 sN Parameter: 1.10 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
 cyclohepta-1,3-diene | dichloromethane | N Parameter: 0.06 sN Parameter: 1.10 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
 cyclohepta-1,3,5-trienyl-Fe(CO)3 | dichloromethane | N Parameter: 3.42 sN Parameter: 0.94 | J. Am. Chem. Soc. 2001, 123, 9500-9512 DOI: 10.1021/ja010890y | |
 cyano(pyridin-1-ium-1-yl)methanide (in DMSO) | DMSO | N Parameter: 25.94 sN Parameter: 0.42 | J. Am. Chem. Soc. 2013, 135, 15216-15224 DOI: 10.1021/ja407885h | |
 cyano((diphenylmethylene)amino)methanide | DMSO | N Parameter: 29.50 sN Parameter: 0.50 | Tetrahedron 2019, 75, 459-463 DOI: 10.1016/j.tet.2018.11.075 | |
 cyanide (in MeCN) | MeCN | N Parameter: 16.27 sN Parameter: 0.70 | Angew. Chem. Int. Ed. 2005, 44, 142-145 DOI: 10.1002/anie.200461640 | |
 Cp2Zr(Me)2 - Dimethylzirconocene | dichloromethane | N Parameter: 4.35 sN Parameter: 1.09 | Chem. Eur. J. 2016, 22, 11196-11200 DOI: 10.1002/chem.201602452 | |
 Cp2Zr(CH2Ph)2 - Dibenzylzirconocene | dichloromethane | N Parameter: 5.10 sN Parameter: 1.03 | Chem. Eur. J. 2016, 22, 11196-11200 DOI: 10.1002/chem.201602452 | |
 Cp2Ti(CH2Ph)2 - Dibenzyltitanocene | dichloromethane | N Parameter: 4.38 sN Parameter: 1.00 | Chem. Eur. J. 2016, 22, 11196-11200 DOI: 10.1002/chem.201602452 | |
 chloro(phenylsulfonyl)methanide (in DMSO) | DMSO | N Parameter: 28.27 sN Parameter: 0.42 | J. Org. Chem. 2017, 82, 2011-2017 DOI: 10.1021/acs.joc.6b02844 | |
 chloro(phenylsulfonyl)methanide (in DMF) | DMF | N Parameter: 26.64 sN Parameter: 0.64 | Chem. Eur. J. 2008, 14, 6108-6118 DOI: 10.1002/chem.200800329 | |
 chloro((4-nitrophenyl)sulfonyl)methanide (in DMSO) | DMSO | N Parameter: 24.88 sN Parameter: 0.49 | J. Org. Chem. 2017, 82, 2011-2017 DOI: 10.1021/acs.joc.6b02844 | |
 chloro((4-cyanophenyl)sulfonyl)methanide (in DMSO) | DMSO | N Parameter: 25.59 sN Parameter: 0.51 | J. Org. Chem. 2017, 82, 2011-2017 DOI: 10.1021/acs.joc.6b02844 | |
 chloro((4-chlorophenyl)sulfonyl)methanide (in DMSO) | DMSO | N Parameter: 26.90 sN Parameter: 0.45 | J. Org. Chem. 2017, 82, 2011-2017 DOI: 10.1021/acs.joc.6b02844 | |
 buta-1,3-diene | dichloromethane | N Parameter: -0.87 sN Parameter: 1.00 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
 bromo(phenylsulfonyl)methanide (in DMSO) | DMSO | N Parameter: 23.90 sN Parameter: 0.62 | J. Org. Chem. 2017, 82, 2011-2017 DOI: 10.1021/acs.joc.6b02844 | |
 bis(phenylsulfonyl)methanide (in DMSO) | DMSO | N Parameter: 15.68 sN Parameter: 0.74 | Angew. Chem. Int. Ed. 2016, 55, 12845-12848 DOI: 10.1002/anie.201605616 | |
 bis(4-nitrophenyl)methanide (in DMSO) | DMSO | N Parameter: 19.92 sN Parameter: 0.67 | ARKIVOC 2008, (x), 37-53 DOI: 10.3998/ark.5550190.0009.a05 | |
 beta-(trimethylsilyl)styrene | dichloromethane | N Parameter: -0.43 sN Parameter: 1.06 | Chem. Eur. J. 2014, 20, 1103-1110 DOI: 10.1002/chem.201303215 | |
 benzyl isocyanide | dichloromethane | N Parameter: 4.90 sN Parameter: 0.74 | Angew. Chem. Int. Ed. 2007, 46, 3563-3566 DOI: 10.1002/anie.200605205 | |
 benzyl (1-phenylvinyl)carbamate | MeCN | N Parameter: 6.21 sN Parameter: 0.87 | Chem. Eur. J. 2012, 18, 5732-5740 DOI: 10.1002/chem.201103519 | |
 barbiturate anion (in DMSO) | DMSO | N Parameter: 15.59 sN Parameter: 0.80 | J. Org. Chem. 2017, 82, 8476-8488 DOI: 10.1021/acs.joc.7b01223 | |
 azulene | MeCN | N Parameter: 6.66 sN Parameter: 1.02 | Eur. J. Org. Chem. 2009, , 1202-1206 DOI: 10.1002/ejoc.200801099 | |
 anisole | dichloromethane | N Parameter: -1.18 sN Parameter: 1.20 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
 anion of triethyl methanetricarboxylate (in DMSO) | DMSO | N Parameter: 15.33 sN Parameter: 0.72 | Eur. J. Org. Chem. 2016, , 1841-1848 DOI: 10.1002/ejoc.201600107 | |
 anion of phenylnitromethane (in H2O) | water | N Parameter: 12.05 sN Parameter: 0.53 | J. Org. Chem. 2004, 69, 7565-7576 DOI: 10.1021/jo048773j | |
 anion of phenylnitromethane (in DMSO) | DMSO | N Parameter: 18.29 sN Parameter: 0.71 | J. Org. Chem. 2004, 69, 7565-7576 DOI: 10.1021/jo048773j | |
 anion of phenylnitromethane (in 91M9AN) | MeOH-MeCN mix | N Parameter: 12.51 sN Parameter: 0.67 | Eur. J. Org. Chem. 2006, , 2530-2537 DOI: 10.1002/ejoc.200500769 | |
 anion of p-tolylnitromethane (in water) | water | N Parameter: 13.09 sN Parameter: 0.50 | J. Org. Chem. 2004, 69, 7565-7576 DOI: 10.1021/jo048773j | |
 anion of p-tolylnitromethane (in DMSO) | DMSO | N Parameter: 18.31 sN Parameter: 0.76 | J. Org. Chem. 2004, 69, 7565-7576 DOI: 10.1021/jo048773j | |
 anion of p-tolylnitromethane (in 91M9AN) | MeOH-MeCN mix | N Parameter: 13.58 sN Parameter: 0.64 | Eur. J. Org. Chem. 2006, , 2530-2537 DOI: 10.1002/ejoc.200500769 | |
 anion of nitromethane (in water) | water | N Parameter: 12.06 sN Parameter: 0.53 | J. Org. Chem. 2004, 69, 7565-7576 DOI: 10.1021/jo048773j | |
 anion of nitromethane (in DMSO) | DMSO | N Parameter: 20.71 sN Parameter: 0.60 | J. Org. Chem. 2004, 69, 7565-7576 DOI: 10.1021/jo048773j | |
 anion of nitromethane (in 91M9AN) | MeOH-MeCN mix | N Parameter: 14.02 sN Parameter: 0.61 | Eur. J. Org. Chem. 2006, , 2530-2537 DOI: 10.1002/ejoc.200500769 | |
 anion of nitroethane (in water) | water | N Parameter: 11.25 sN Parameter: 0.52 | J. Org. Chem. 2004, 69, 7565-7576 DOI: 10.1021/jo048773j | |
 anion of nitroethane (in DMSO) | DMSO | N Parameter: 21.54 sN Parameter: 0.62 | J. Org. Chem. 2003, 68, 6880-6886 DOI: 10.1021/jo0344182 | |
 anion of nitroethane (in DMF) | DMF | N Parameter: 22.21 sN Parameter: 0.48 | Chem. Eur. J. 2008, 14, 6108-6118 DOI: 10.1002/chem.200800329 | |
 anion of nitroethane (in 91M9AN) | MeOH-MeCN mix | N Parameter: 13.41 sN Parameter: 0.67 | Eur. J. Org. Chem. 2006, , 2530-2537 DOI: 10.1002/ejoc.200500769 | |
 anion of N,N-diethyl-3-oxo-3-phenylpropanoate (in DMSO) | DMSO | N Parameter: 19.28 sN Parameter: 0.65 | Chem. Eur. J. 2015, 21, 875-884 DOI: 10.1002/chem.201404500 | |
 anion of Meldrums acid (in DMSO) | DMSO | N Parameter: 13.91 sN Parameter: 0.86 | Angew. Chem. Int. Ed. 2002, 41, 91-95 DOI: 10.1002/1521-3773(20020104)41:1<91::AID-ANIE91>3.0.CO;2-P | |
 anion of Meldrum's acid (in water) | water | N Parameter: 12.06 sN Parameter: 0.66 | J. Am. Chem. Soc. 2003, 125, 12980-12986 DOI: 10.1021/ja036838e | |
 anion of malononitrile (in water) | water | N Parameter: 19.50 sN Parameter: 0.55 | J. Am. Chem. Soc. 2003, 125, 12980-12986 DOI: 10.1021/ja036838e | |
 anion of malononitrile (in DMSO) | DMSO | N Parameter: 19.36 sN Parameter: 0.67 | Angew. Chem. Int. Ed. 2002, 41, 91-95 DOI: 10.1002/1521-3773(20020104)41:1<91::AID-ANIE91>3.0.CO;2-P | |
 anion of malononitrile (in 91M9AN) | MeOH-MeCN mix | N Parameter: 18.21 sN Parameter: 0.69 | Eur. J. Org. Chem. 2006, , 2530-2537 DOI: 10.1002/ejoc.200500769 | |
 anion of ethyl cyanoacetate (in water) | water | N Parameter: 15.57 sN Parameter: 0.58 | J. Am. Chem. Soc. 2003, 125, 12980-12986 DOI: 10.1021/ja036838e | |
 anion of ethyl cyanoacetate (in DMSO) | DMSO | N Parameter: 19.62 sN Parameter: 0.67 | Angew. Chem. Int. Ed. 2002, 41, 91-95 DOI: 10.1002/1521-3773(20020104)41:1<91::AID-ANIE91>3.0.CO;2-P | |
 anion of ethyl cyanoacetate (in 91M9AN) | MeOH-MeCN mix | N Parameter: 18.59 sN Parameter: 0.65 | Eur. J. Org. Chem. 2006, , 2530-2537 DOI: 10.1002/ejoc.200500769 | |
 anion of ethyl acetylacetate (in water) | water | N Parameter: 15.99 sN Parameter: 0.62 | J. Am. Chem. Soc. 2003, 125, 12980-12986 DOI: 10.1021/ja036838e | |
 anion of ethyl acetylacetate (in DMSO) | DMSO | N Parameter: 18.82 sN Parameter: 0.69 | Angew. Chem. Int. Ed. 2002, 41, 91-95 DOI: 10.1002/1521-3773(20020104)41:1<91::AID-ANIE91>3.0.CO;2-P | |
 anion of ethyl 3-oxo-3-phenylpropanoate (in DMSO) | DMSO | N Parameter: 17.52 sN Parameter: 0.74 | Chem. Eur. J. 2015, 21, 875-884 DOI: 10.1002/chem.201404500 | |
 anion of ethyl 2-nitroacetate (in DMSO) | DMSO | N Parameter: 15.24 sN Parameter: 0.74 | Chem. Eur. J. 2015, 21, 875-884 DOI: 10.1002/chem.201404500 | |
 anion of ethyl 2-cyano-2-phenylacetate (in DMSO) | DMSO | N Parameter: 15.85 sN Parameter: 1.04 | Eur. J. Org. Chem. 2017, , 1196-1202 DOI: 10.1002/ejoc.201601513 | |
 anion of dimethyl malonate (in 91M9AN) | MeOH-MeCN mix | N Parameter: 18.24 sN Parameter: 0.64 | Eur. J. Org. Chem. 2006, , 2530-2537 DOI: 10.1002/ejoc.200500769 | |
 anion of dimedone (in water) | water | N Parameter: 11.77 sN Parameter: 0.63 | J. Am. Chem. Soc. 2003, 125, 12980-12986 DOI: 10.1021/ja036838e | |
 anion of dimedone (in DMSO) | DMSO | N Parameter: 16.27 sN Parameter: 0.77 | Angew. Chem. Int. Ed. 2002, 41, 91-95 DOI: 10.1002/1521-3773(20020104)41:1<91::AID-ANIE91>3.0.CO;2-P | |
 anion of diethyl methylmalonate (in water) | water | N Parameter: 17.68 sN Parameter: 0.50 | J. Am. Chem. Soc. 2003, 125, 12980-12986 DOI: 10.1021/ja036838e | |
 anion of diethyl methylmalonate (in DMSO) | DMSO | N Parameter: 21.13 sN Parameter: 0.68 | J. Am. Chem. Soc. 2003, 125, 12980-12986 DOI: 10.1021/ja036838e | |
 anion of diethyl malonate (in water) | water | N Parameter: 16.15 sN Parameter: 0.66 | J. Am. Chem. Soc. 2003, 125, 12980-12986 DOI: 10.1021/ja036838e | |
 anion of diethyl malonate (in DMSO) | DMSO | N Parameter: 20.22 sN Parameter: 0.65 | Angew. Chem. Int. Ed. 2002, 41, 91-95 DOI: 10.1002/1521-3773(20020104)41:1<91::AID-ANIE91>3.0.CO;2-P | |
 anion of diethyl 2-phenylmalonate (in DMSO) | DMSO | N Parameter: 15.93 sN Parameter: 0.99 | Eur. J. Org. Chem. 2016, , 1841-1848 DOI: 10.1002/ejoc.201600107 | |
 anion of diethyl 2-cyclohexylmalonate (in DMSO) | DMSO | N Parameter: 19.23 sN Parameter: 0.67 | Eur. J. Org. Chem. 2016, , 1841-1848 DOI: 10.1002/ejoc.201600107 | |
 anion of diethyl 2-butylmalonate (in DMSO) | DMSO | N Parameter: 23.00 sN Parameter: 0.55 | Eur. J. Org. Chem. 2016, , 1841-1848 DOI: 10.1002/ejoc.201600107 | |
 anion of diethyl 2-(p-anisyl)malonate (in DMSO) | DMSO | N Parameter: 16.73 sN Parameter: 0.91 | Eur. J. Org. Chem. 2016, , 1841-1848 DOI: 10.1002/ejoc.201600107 | |
 anion of diethyl 2-(isopropyl)malonate (in DMSO) | DMSO | N Parameter: 18.74 sN Parameter: 0.71 | Eur. J. Org. Chem. 2016, , 1841-1848 DOI: 10.1002/ejoc.201600107 | |
 anion of diethyl 2-(benzoyl)malonate (in DMSO) | DMSO | N Parameter: 13.63 sN Parameter: 0.80 | Eur. J. Org. Chem. 2016, , 1841-1848 DOI: 10.1002/ejoc.201600107 | |
 anion of diethyl 2-(acetyl)malonate (in DMSO) | DMSO | N Parameter: 13.83 sN Parameter: 0.84 | Eur. J. Org. Chem. 2016, , 1841-1848 DOI: 10.1002/ejoc.201600107 | |
 anion of diethyl 2-(4-nitrophenyl)malonate (in DMSO) | DMSO | N Parameter: 14.94 sN Parameter: 0.96 | Eur. J. Org. Chem. 2016, , 1841-1848 DOI: 10.1002/ejoc.201600107 | |
 anion of benzyl trifluoromethyl sulfone (in DMSO) | DMSO | N Parameter: 18.67 sN Parameter: 0.68 | J. Am. Chem. Soc. 2007, 129, 9753-9761 DOI: 10.1021/ja072135b | |
 anion of acetylacetone (in water) | water | N Parameter: 13.73 sN Parameter: 0.64 | J. Am. Chem. Soc. 2003, 125, 12980-12986 DOI: 10.1021/ja036838e | |
 anion of acetylacetone (in DMSO) | DMSO | N Parameter: 17.64 sN Parameter: 0.73 | Angew. Chem. Int. Ed. 2002, 41, 91-95 DOI: 10.1002/1521-3773(20020104)41:1<91::AID-ANIE91>3.0.CO;2-P | |
 anion of 5-phenyl Meldrum's acid (in DMSO) | DMSO | N Parameter: 11.54 sN Parameter: 0.95 | Eur. J. Org. Chem. 2016, , 1841-1848 DOI: 10.1002/ejoc.201600107 | |
 anion of 5-(p-anisyl) Meldrum's acid (in DMSO) | DMSO | N Parameter: 12.35 sN Parameter: 0.90 | Eur. J. Org. Chem. 2016, , 1841-1848 DOI: 10.1002/ejoc.201600107 | |
 anion of 4-nitrobenzyl-CN (in MeCN) | MeCN | N Parameter: 20.10 sN Parameter: 0.71 | J. Am. Chem. Soc. 2020, 142, 8383-8402 DOI: 10.1021/jacs.0c01960 | |
 anion of 4-nitrobenzyl-CN (in DMSO) | DMSO | N Parameter: 19.67 sN Parameter: 0.68 | J. Org. Chem. 2009, 74, 75-81 DOI: 10.1021/jo802241x | |
 anion of 4-nitrobenzyl trifluoromethyl sulfone (in DMSO) | DMSO | N Parameter: 14.49 sN Parameter: 0.86 | J. Am. Chem. Soc. 2007, 129, 9753-9761 DOI: 10.1021/ja072135b | |
 anion of 4-nitrobenzyl trifluoromethyl sulfone (in 91M9AN) | MeOH-MeCN mix | N Parameter: 18.24 sN Parameter: 0.66 | J. Am. Chem. Soc. 2007, 129, 9753-9761 DOI: 10.1021/ja072135b | |
 anion of 4-methylbenzyl trifluoromethyl sulfone (in DMSO) | DMSO | N Parameter: 19.35 sN Parameter: 0.67 | J. Am. Chem. Soc. 2007, 129, 9753-9761 DOI: 10.1021/ja072135b | |
 anion of 4-cyanobenzyl-CN (in DMSO) | DMSO | N Parameter: 25.11 sN Parameter: 0.54 | J. Org. Chem. 2009, 74, 75-81 DOI: 10.1021/jo802241x | |
 anion of 4-cyanobenzyl trifluoromethyl sulfone (in MeCN) | MeCN | N Parameter: 15.62 sN Parameter: 0.99 | J. Am. Chem. Soc. 2020, 142, 8383-8402 DOI: 10.1021/jacs.0c01960 | |
 anion of 4-cyanobenzyl trifluoromethyl sulfone (in DMSO) | DMSO | N Parameter: 16.28 sN Parameter: 0.75 | J. Am. Chem. Soc. 2007, 129, 9753-9761 DOI: 10.1021/ja072135b | |
 anion of 4-cyanobenzyl trifluoromethyl sulfone (in 91M9AN) | MeOH-MeCN mix | N Parameter: 19.49 sN Parameter: 0.63 | J. Am. Chem. Soc. 2007, 129, 9753-9761 DOI: 10.1021/ja072135b | |
 anion of 4-(trifluoromethyl)benzyl-CN (in DMSO) | DMSO | N Parameter: 27.28 sN Parameter: 0.50 | J. Org. Chem. 2009, 74, 75-81 DOI: 10.1021/jo802241x | |
 anion of 4-(trifluoromethyl)benzyl trifluoromethyl sulfone (in MeCN) | MeCN | N Parameter: 16.15 sN Parameter: 0.99 | J. Am. Chem. Soc. 2020, 142, 8383-8402 DOI: 10.1021/jacs.0c01960 | |
 anion of 4-(trifluoromethyl)benzyl trifluoromethyl sulfone (in DMSO) | DMSO | N Parameter: 17.33 sN Parameter: 0.74 | J. Am. Chem. Soc. 2007, 129, 9753-9761 DOI: 10.1021/ja072135b | |
 anion of 4-(trifluoromethyl)benzyl trifluoromethyl sulfone (in 91M9AN) | MeOH-MeCN mix | N Parameter: 20.72 sN Parameter: 0.58 | J. Am. Chem. Soc. 2007, 129, 9753-9761 DOI: 10.1021/ja072135b | |
 anion of 3-oxo-3-phenylpropanenitrile (in DMSO) | DMSO | N Parameter: 16.55 sN Parameter: 0.78 | Chem. Eur. J. 2015, 21, 875-884 DOI: 10.1002/chem.201404500 | |
 anion of 3-methyl acetylacetone (in water) | water | N Parameter: 14.33 sN Parameter: 0.69 | J. Am. Chem. Soc. 2003, 125, 12980-12986 DOI: 10.1021/ja036838e | |
 anion of 3-methyl acetylacetone (in DMSO) | DMSO | N Parameter: 18.38 sN Parameter: 0.72 | J. Am. Chem. Soc. 2003, 125, 12980-12986 DOI: 10.1021/ja036838e | |
 anion of 2-phenylmalononitrile (in DMSO) | DMSO | N Parameter: 15.58 sN Parameter: 1.00 | Eur. J. Org. Chem. 2017, , 1196-1202 DOI: 10.1002/ejoc.201601513 | |
 anion of 2-phenyl-propionitrile (in DMSO) | DMSO | N Parameter: 28.95 sN Parameter: 0.58 | J. Org. Chem. 2009, 74, 75-81 DOI: 10.1021/jo802241x | |
 anion of 2-phenyl-2-(phenylsulfonyl)acetonitrile (in DMSO) | DMSO | N Parameter: 15.97 sN Parameter: 0.72 | Eur. J. Org. Chem. 2017, , 1196-1202 DOI: 10.1002/ejoc.201601513 | |
 anion of 2-nitropropane (in water) | water | N Parameter: 10.69 sN Parameter: 0.56 | J. Org. Chem. 2004, 69, 7565-7576 DOI: 10.1021/jo048773j | |
 anion of 2-nitropropane (in DMSO) | DMSO | N Parameter: 20.61 sN Parameter: 0.69 | J. Org. Chem. 2004, 69, 7565-7576 DOI: 10.1021/jo048773j | |
 anion of 2-nitropropane (in 91M9AN) | MeOH-MeCN mix | N Parameter: 12.20 sN Parameter: 0.71 | Eur. J. Org. Chem. 2006, , 2530-2537 DOI: 10.1002/ejoc.200500769 | |
 anion of 2-nitro-1-phenylethan-1-one (in DMSO) | DMSO | N Parameter: 13.91 sN Parameter: 0.76 | Chem. Eur. J. 2015, 21, 875-884 DOI: 10.1002/chem.201404500 | |
 anion of 2-(4-NO2-C6H4)propionitrile (in DMSO) | DMSO | N Parameter: 19.61 sN Parameter: 0.60 | J. Org. Chem. 2009, 74, 75-81 DOI: 10.1021/jo802241x | |
 anion of 2-(4-CN-C6H4)propionitrile (in DMSO) | DMSO | N Parameter: 25.35 sN Parameter: 0.56 | J. Org. Chem. 2009, 74, 75-81 DOI: 10.1021/jo802241x | |
 anion of 1-phenylpropan-2-one (in DMSO) | DMSO | N Parameter: 24.99 sN Parameter: 0.60 | Chem. Eur. J. 2015, 21, 875-884 DOI: 10.1002/chem.201404500 | |
 anion of 1-phenylbutane-1,3-dione (in DMSO) | DMSO | N Parameter: 16.03 sN Parameter: 0.86 | Chem. Eur. J. 2015, 21, 875-884 DOI: 10.1002/chem.201404500 | |
 anion of 1-phenyl-2-(phenylsulfonyl)ethanone (in DMSO) | DMSO | N Parameter: 17.19 sN Parameter: 0.56 | Chem. Eur. J. 2015, 21, 875-884 DOI: 10.1002/chem.201404500 | |
 anion of 1,3-diphenylpropane-1,3-dione (in DMSO) | DMSO | N Parameter: 17.46 sN Parameter: 0.65 | Chem. Eur. J. 2015, 21, 875-884 DOI: 10.1002/chem.201404500 | |
 anion of 1,2-diphenylethanone (in DMSO) | DMSO | N Parameter: 23.15 sN Parameter: 0.60 | Chem. Eur. J. 2015, 21, 875-884 DOI: 10.1002/chem.201404500 | |
 anion of 1,2,3-triphenylpropane-1,3-dione (in DMSO) | DMSO | N Parameter: 14.99 sN Parameter: 0.83 | Eur. J. Org. Chem. 2017, , 1196-1202 DOI: 10.1002/ejoc.201601513 | |
 anion of (phenylmethylenedisulfonyl)dibenzene (in DMSO) | DMSO | N Parameter: 15.07 sN Parameter: 0.79 | Eur. J. Org. Chem. 2017, , 1196-1202 DOI: 10.1002/ejoc.201601513 | |
 anion of (benzylsulfonyl)benzene (in DMSO) | DMSO | N Parameter: 25.77 sN Parameter: 0.56 | Chem. Eur. J. 2015, 21, 875-884 DOI: 10.1002/chem.201404500 | |
 anion of (4-NO2-C6H4)CH2SO2Ph (in MeCN) | MeCN | N Parameter: 19.90 sN Parameter: 0.66 | J. Am. Chem. Soc. 2020, 142, 8383-8402 DOI: 10.1021/jacs.0c01960 | |
 anion of (4-NO2-C6H4)CH2SO2Ph (in DMSO) | DMSO | N Parameter: 18.50 sN Parameter: 0.75 | Org. Biomol. Chem. 2008, 6, 3052-3058 DOI: 10.1039/b805604h | |
 anion of (4-nitrophenyl)nitromethane (in water) | water | N Parameter: 13.58 sN Parameter: 0.52 | J. Org. Chem. 2004, 69, 7565-7576 DOI: 10.1021/jo048773j | |
 anion of (4-nitrophenyl)nitromethane (in DMSO) | DMSO | N Parameter: 16.29 sN Parameter: 0.75 | J. Org. Chem. 2004, 69, 7565-7576 DOI: 10.1021/jo048773j | |
 anion of (4-nitrophenyl)nitromethane (in 91M9AN) | MeOH-MeCN mix | N Parameter: 14.05 sN Parameter: 0.72 | Eur. J. Org. Chem. 2006, , 2530-2537 DOI: 10.1002/ejoc.200500769 | |
 anion of (4-cyanophenyl)nitromethane (in water) | water | N Parameter: 13.23 sN Parameter: 0.52 | J. Org. Chem. 2004, 69, 7565-7576 DOI: 10.1021/jo048773j | |
 anion of (4-cyanophenyl)nitromethane (in DMSO) | DMSO | N Parameter: 16.96 sN Parameter: 0.73 | J. Org. Chem. 2004, 69, 7565-7576 DOI: 10.1021/jo048773j | |
 anion of (4-cyanophenyl)nitromethane (in 91M9AN) | MeOH-MeCN mix | N Parameter: 13.92 sN Parameter: 0.74 | Eur. J. Org. Chem. 2006, , 2530-2537 DOI: 10.1002/ejoc.200500769 | |
 anion of (4-CN-C6H4)CH2SO2Ph (in DMSO) | DMSO | N Parameter: 22.60 sN Parameter: 0.57 | Org. Biomol. Chem. 2008, 6, 3052-3058 DOI: 10.1039/b805604h | |
 anion of (4-CF3-C6H4)CH2SO2Ph (in DMSO) | DMSO | N Parameter: 24.30 sN Parameter: 0.51 | Org. Biomol. Chem. 2008, 6, 3052-3058 DOI: 10.1039/b805604h | |
 anion of (3-nitrophenyl)nitromethane (in water) | water | N Parameter: 14.25 sN Parameter: 0.46 | J. Org. Chem. 2004, 69, 7565-7576 DOI: 10.1021/jo048773j | |
 anion of (3-nitrophenyl)nitromethane (in DMSO) | DMSO | N Parameter: 18.06 sN Parameter: 0.71 | J. Org. Chem. 2004, 69, 7565-7576 DOI: 10.1021/jo048773j | |
 anion of (3-nitrophenyl)nitromethane (in 91M9AN) | MeOH-MeCN mix | N Parameter: 14.75 sN Parameter: 0.71 | Eur. J. Org. Chem. 2006, , 2530-2537 DOI: 10.1002/ejoc.200500769 | |
 alpha-methylstyrene | dichloromethane | N Parameter: 2.35 sN Parameter: 1.00 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
 alpha-(trimethylsilyl)styrene | dichloromethane | N Parameter: -1.13 sN Parameter: 1.46 | Chem. Eur. J. 2014, 20, 1103-1110 DOI: 10.1002/chem.201303215 | |
 alpha-(pentamethyldisilyl)styrene | dichloromethane | N Parameter: 0.61 sN Parameter: 1.01 | Chem. Eur. J. 2014, 20, 1103-1110 DOI: 10.1002/chem.201303215 | |
 alpha-(N-piperidino)styrene (in MeCN) | MeCN | N Parameter: 11.60 sN Parameter: 0.80 | Chem. Eur. J. 2018, 24, 5901-5910 DOI: 10.1002/chem.201705962 | |
 alpha-(N-morpholino)styrene (in MeCN) | MeCN | N Parameter: 10.30 sN Parameter: 0.80 | Chem. Eur. J. 2018, 24, 5901-5910 DOI: 10.1002/chem.201705962 | |
 alpha-(N-morpholino)styrene | dichloromethane | N Parameter: 9.96 sN Parameter: 0.79 | Chem. Eur. J. 2003, 9, 2209-2218 DOI: 10.1002/chem.200204666 | |
 allyltris(trimethylsilyl)silane | dichloromethane | N Parameter: 1.95 sN Parameter: 0.98 | Org. Lett. 2010, 12, 5206-5209 DOI: 10.1021/ol102220e | |
 allyltriphenylstannane | dichloromethane | N Parameter: 3.09 sN Parameter: 0.90 | J. Am. Chem. Soc. 2001, 123, 9500-9512 DOI: 10.1021/ja010890y | |
 allyltriphenylsilane | dichloromethane | N Parameter: -0.13 sN Parameter: 1.21 | J. Am. Chem. Soc. 2001, 123, 9500-9512 DOI: 10.1021/ja010890y | |
 allyltriphenylgermane | dichloromethane | N Parameter: 1.20 sN Parameter: 0.90 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
 allyltrimethylsilane | dichloromethane | N Parameter: 1.68 sN Parameter: 1.00 | J. Am. Chem. Soc. 2012, 134, 13902-13911 DOI: 10.1021/ja306522b | |
 allyltriisopropylsilane | dichloromethane | N Parameter: 2.04 sN Parameter: 0.95 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
 allyltrihexylsilane | dichloromethane | N Parameter: 2.11 sN Parameter: 0.95 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
 allyltriethylsilane | dichloromethane | N Parameter: 1.93 sN Parameter: 0.95 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
 allyltributylstannane | dichloromethane | N Parameter: 5.46 sN Parameter: 0.89 | J. Am. Chem. Soc. 2001, 123, 9500-9512 DOI: 10.1021/ja010890y | |
 allyltri-n-butylsilane | dichloromethane | N Parameter: 2.09 sN Parameter: 0.95 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
 allylchlorodimethylsilane | dichloromethane | N Parameter: -0.57 sN Parameter: 1.06 | J. Am. Chem. Soc. 2012, 134, 13902-13911 DOI: 10.1021/ja306522b | |
 allyl-tert.butyl-dimethylsilane | dichloromethane | N Parameter: 1.80 sN Parameter: 0.95 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
 7-azaindole | MeCN | N Parameter: 3.87 sN Parameter: 1.10 | J. Org. Chem. 2006, 71, 9088-9095 DOI: 10.1021/jo0614339 | |
 6-methyl-3-(phenyl-l3-iodanylidene)dihydro-2H-pyran-2,4(3H)-dione (in CH2Cl2) | dichloromethane | N Parameter: 4.67 sN Parameter: 0.92 | J. Am. Chem. Soc. 2016, 138, 10304-10313 DOI: 10.1021/jacs.6b05768 | |
 6-(julolidin-9-yl)fulvene | MeCN | N Parameter: 7.79 sN Parameter: 1.06 | Eur. J. Org. Chem. 2009, , 1202-1206 DOI: 10.1002/ejoc.200801099 | |
 6-(4-dimethylamino-phenyl)fulvene | MeCN | N Parameter: 6.72 sN Parameter: 0.87 | Eur. J. Org. Chem. 2009, , 1202-1206 DOI: 10.1002/ejoc.200801099 | |
 6,6-dimethylfulvene | MeCN | N Parameter: 3.93 sN Parameter: 0.88 | Eur. J. Org. Chem. 2009, , 1202-1206 DOI: 10.1002/ejoc.200801099 | |
 5-methyl-indole | MeCN | N Parameter: 6.00 sN Parameter: 1.10 | J. Org. Chem. 2006, 71, 9088-9095 DOI: 10.1021/jo0614339 | |
 5-methoxy-indole | dichloromethane | N Parameter: 6.22 sN Parameter: 1.12 | J. Org. Chem. 2006, 71, 9088-9095 DOI: 10.1021/jo0614339 | |
 5-methoxy-2-methylindole | MeCN | N Parameter: 7.26 sN Parameter: 1.10 | J. Org. Chem. 2006, 71, 9088-9095 DOI: 10.1021/jo0614339 | |
 5-hydroxy-indole | MeCN | N Parameter: 6.44 sN Parameter: 1.10 | J. Org. Chem. 2006, 71, 9088-9095 DOI: 10.1021/jo0614339 | |
 5-cyano-indole | MeCN | N Parameter: 2.83 sN Parameter: 1.10 | J. Org. Chem. 2006, 71, 9088-9095 DOI: 10.1021/jo0614339 | |
 5-chloro-indole | MeCN | N Parameter: 4.42 sN Parameter: 1.10 | J. Org. Chem. 2006, 71, 9088-9095 DOI: 10.1021/jo0614339 | |
 5-chloro-2-methylindole | MeCN | N Parameter: 6.08 sN Parameter: 1.10 | J. Org. Chem. 2006, 71, 9088-9095 DOI: 10.1021/jo0614339 | |
 5-bromo-indole | MeCN | N Parameter: 4.38 sN Parameter: 1.10 | J. Org. Chem. 2006, 71, 9088-9095 DOI: 10.1021/jo0614339 | |
 5-benzyl-2,2-dimethyl-4,6-dioxo-1,3-dioxan-5-ide (in DMSO) | DMSO | N Parameter: 15.02 sN Parameter: 0.75 | Chem. Eur. J. 2014, 20, 11069-11077 DOI: 10.1002/chem.201403161 | |
 5-amino-indole | MeCN | N Parameter: 7.22 sN Parameter: 1.10 | J. Org. Chem. 2006, 71, 9088-9095 DOI: 10.1021/jo0614339 | |
 5-(tris(pentafluorophenyl)siloxy)-2,3-dihydrofuran | dichloromethane | N Parameter: 8.16 sN Parameter: 0.67 | Eur. J. Org. Chem. 2005, , 1760-1764 DOI: 10.1002/ejoc.200400706 | |
 5-(triphenylsiloxy)-2,3-dihydrofuran | dichloromethane | N Parameter: 11.28 sN Parameter: 0.91 | Eur. J. Org. Chem. 2005, , 1760-1764 DOI: 10.1002/ejoc.200400706 | |
 5-(trimethylsiloxy)-2,3-dihydrofuran (in MeCN) | MeCN | N Parameter: 12.34 sN Parameter: 0.72 | J. Am. Chem. Soc. 2013, 135, 6579-6587 DOI: 10.1021/ja401106x | |
 5-(trimethylsiloxy)-2,3-dihydrofuran | dichloromethane | N Parameter: 12.56 sN Parameter: 0.70 | J. Am. Chem. Soc. 2001, 123, 9500-9512 DOI: 10.1021/ja010890y | |
 5-(tert-Butyl)-1,3-diisopropyl-4-methylene-4,5-dihydro-1H-1,2,3-triazol-3-ium-5-ide (in THF) | THF | N Parameter: 31.92 sN Parameter: 0.52 | Angew. Chem. Int. Ed. 2023, 62, e202309790 DOI: 10.1002/anie.202309790 | |
 5-(4-cyanobenzyl)-2,2-dimethyl-4,6-dioxo-1,3-dioxan-5-ide (in DMSO) | DMSO | N Parameter: 13.79 sN Parameter: 0.86 | Chem. Eur. J. 2014, 20, 11069-11077 DOI: 10.1002/chem.201403161 | |
 5-(4-(methoxy)benzyl)-2,2-dimethyl-4,6-dioxo-1,3-dioxan-5-ide (in DMSO) | DMSO | N Parameter: 15.13 sN Parameter: 0.75 | Chem. Eur. J. 2014, 20, 11069-11077 DOI: 10.1002/chem.201403161 | |
 5-(4-(dimethylamino)benzyl)-2,2-dimethyl-4,6-dioxo-1,3-dioxan-5-ide (in DMSO) | DMSO | N Parameter: 14.48 sN Parameter: 0.86 | Chem. Eur. J. 2014, 20, 11069-11077 DOI: 10.1002/chem.201403161 | |
 4-methyl-penta-1,3-diene | dichloromethane | N Parameter: 2.60 sN Parameter: 1.00 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
 4-methyl-N-(2-phenylethynyl)-N-(phenylmethyl)benzenesulfonamide | dichloromethane | N Parameter: 3.85 sN Parameter: 0.84 | Angew. Chem. Int. Ed. 2014, 53, 4968-4971 DOI: 10.1002/anie.201402055 | |
 4-methyl-5-(trimethylsiloxy)-2,3-dihydrofuran | dichloromethane | N Parameter: 11.50 sN Parameter: 0.91 | Eur. J. Org. Chem. 2004, , 2791-2796 DOI: 10.1002/ejoc.200400134 | |
 4-methoxy-indole | MeCN | N Parameter: 5.41 sN Parameter: 1.10 | J. Org. Chem. 2006, 71, 9088-9095 DOI: 10.1021/jo0614339 | |
 4-cyanophenyl isocyanide | dichloromethane | N Parameter: 3.57 sN Parameter: 0.72 | Angew. Chem. Int. Ed. 2007, 46, 3563-3566 DOI: 10.1002/anie.200605205 | |
 4-(trimethylsilyl)but-1-en-3-ynyl-Co2(CO)6 | dichloromethane | N Parameter: -1.11 sN Parameter: 0.92 | J. Am. Chem. Soc. 2001, 123, 9500-9512 DOI: 10.1021/ja010890y | |
 4-(trimethylsilyl)but-1-en-3-ynyl-Co2(CO)5(PPh3) | dichloromethane | N Parameter: -0.76 sN Parameter: 0.90 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
 4-(bis(trimethylsiloxy)amino)pent-4-enoic acid methyl ester | dichloromethane | N Parameter: 3.84 sN Parameter: 0.87 | J. Org. Chem. 2001, 66, 3196-3200 DOI: 10.1021/jo0015927 | |
 4,4,5,5-tetramethyl-2-prop-2-enyl-1,3,2-dioxaborolane (in CH2Cl2) | dichloromethane | N Parameter: -0.64 sN Parameter: 1.10 | J. Am. Chem. Soc. 2017, 139, 15324-15327 DOI: 10.1021/jacs.7b10240 | |
 3-pyrrolidino-1H-indene (in MeCN) | MeCN | N Parameter: 15.27 sN Parameter: 0.93 | Synthesis 2019, 51, 1157-1170 DOI: 10.1055/s-0037-1611634 | |
 3-methylanisole | dichloromethane | N Parameter: 0.13 sN Parameter: 1.27 | J. Am. Chem. Soc. 2001, 123, 9500-9512 DOI: 10.1021/ja010890y | |
 3-methyl-2-(trimethylsiloxy)but-1-ene | dichloromethane | N Parameter: 5.41 sN Parameter: 0.90 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
 3-methyl-2,4-dioxotetrahydro-2H-pyran-3-ide (in DMSO) | DMSO | N Parameter: 14.46 sN Parameter: 0.91 | Eur. J. Org. Chem. 2015, , 7594-7601 DOI: 10.1002/ejoc.201501107 | |
 3-methoxythiophene | MeCN | N Parameter: 3.06 sN Parameter: 1.19 | J. Am. Chem. Soc. 2013, 135, 6317-6324 DOI: 10.1021/ja4017655 | |
 3-mesityl-1-methyl-1H-imidazol-3-ium-2-ide (in THF) | THF | N Parameter: 21.50 sN Parameter: 0.45 | Org. Lett. 2016, 18, 3566-3569 DOI: 10.1021/acs.orglett.6b01525 | |
 3-ethyl-2,4-dimethylpyrrole | MeCN | N Parameter: 11.63 sN Parameter: 0.95 | Eur. J. Org. Chem. 2008, , 2369-2374 DOI: 10.1002/ejoc.200800092 | |
 3-diazoindolin-2-one | dichloromethane | N Parameter: 3.16 sN Parameter: 1.03 | Eur. J. Org. Chem. 2023, 26, e202300005 DOI: 10.1002/ejoc.202300005 | |
 3-acetyl-2-oxotetrahydro-2H-pyran-3-ide (in DMSO) | DMSO | N Parameter: 17.00 sN Parameter: 0.74 | Eur. J. Org. Chem. 2015, , 7594-7601 DOI: 10.1002/ejoc.201501107 | |
 3-(trimethylsilyl)cyclopentene | dichloromethane | N Parameter: 1.77 sN Parameter: 1.10 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
 3-(trimethylsiloxy)-1H-indene (in MeCN) | MeCN | N Parameter: 7.32 sN Parameter: 0.82 | Synthesis 2019, 51, 1157-1170 DOI: 10.1055/s-0037-1611634 | |
 3-(trihexylsilyl)cyclopentene | dichloromethane | N Parameter: 1.98 sN Parameter: 1.10 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
 3-(tert-butyl)-1-methyl-1H-imidazol-3-ium-2-ide (in THF) | THF | N Parameter: 16.54 sN Parameter: 0.47 | Org. Lett. 2016, 18, 3566-3569 DOI: 10.1021/acs.orglett.6b01525 | |
 3-(4-methoxyphenyl)-1-methyl-1H-imidazol-3-ium-2-ide (in THF) | THF | N Parameter: 20.41 sN Parameter: 0.46 | Org. Lett. 2016, 18, 3566-3569 DOI: 10.1021/acs.orglett.6b01525 | |
 3-(2,6-dimethoxyphenyl)-1-methyl-1H-imidazol-3-ium-2-ide (in THF) | THF | N Parameter: 23.00 sN Parameter: 0.46 | Org. Lett. 2016, 18, 3566-3569 DOI: 10.1021/acs.orglett.6b01525 | |
 3,3-dimethyl-2-(trimethylsiloxy)buten | dichloromethane | N Parameter: 3.78 sN Parameter: 0.79 | J. Am. Chem. Soc. 2001, 123, 9500-9512 DOI: 10.1021/ja010890y | |
 3,3,3-trifluoro-2-(trimethylsiloxy)propene | dichloromethane | N Parameter: -2.94 sN Parameter: 1.11 | Org. Lett. 2012, 14, 3990-3993 DOI: 10.1021/ol301766w | |
 2H-benzo[d][1,3]dithiol-2-ide 1,1,3,3-tetraoxide (in DMSO) | DMSO | N Parameter: 16.06 sN Parameter: 0.69 | Angew. Chem. Int. Ed. 2016, 55, 12845-12848 DOI: 10.1002/anie.201605616 | |
 2-thiobarbiturate anion (in DMSO) | DMSO | N Parameter: 14.24 sN Parameter: 0.82 | J. Org. Chem. 2017, 82, 8476-8488 DOI: 10.1021/acs.joc.7b01223 | |
 2-phenyl-allyltrimethylsilane | dichloromethane | N Parameter: 5.38 sN Parameter: 0.89 | Chem. Eur. J. 2014, 20, 1103-1110 DOI: 10.1002/chem.201303215 | |
 2-phenyl-4-(trimethylsilyl)but-1-en-3-ynyl-Co2(CO)6 | dichloromethane | N Parameter: 1.33 sN Parameter: 0.90 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
 2-PhCH(-)CO2Et (in DMSO) | DMSO | N Parameter: 27.54 sN Parameter: 0.57 | Eur. J. Org. Chem. 2013, , 4255-4261 DOI: 10.1002/ejoc.201300265 | |
 2-oxo-2-phenyl-1-(quinolin-1-ium-1-yl)ethan-1-ide (in DMSO) | DMSO | N Parameter: 19.38 sN Parameter: 0.50 | J. Am. Chem. Soc. 2013, 135, 15216-15224 DOI: 10.1021/ja407885h | |
 2-oxo-2-phenyl-1-(pyridin-1-ium-1-yl)ethan-1-ide (in DMSO) | DMSO | N Parameter: 19.46 sN Parameter: 0.58 | J. Am. Chem. Soc. 2013, 135, 15216-15224 DOI: 10.1021/ja407885h | |
 2-oxo-1-(pyridin-1-ium-1-yl)propan-1-ide (in DMSO) | DMSO | N Parameter: 20.24 sN Parameter: 0.60 | J. Am. Chem. Soc. 2013, 135, 15216-15224 DOI: 10.1021/ja407885h | |
 2-methylthiophene | dichloromethane | N Parameter: 1.35 sN Parameter: 0.99 | J. Am. Chem. Soc. 2012, 134, 13902-13911 DOI: 10.1021/ja306522b | |
 2-methylpropene (isobutylene) | dichloromethane | N Parameter: 1.11 sN Parameter: 0.98 | J. Am. Chem. Soc. 2001, 123, 9500-9512 DOI: 10.1021/ja010890y | |
 2-methylindole | MeCN | N Parameter: 6.91 sN Parameter: 1.10 | J. Org. Chem. 2006, 71, 9088-9095 DOI: 10.1021/jo0614339 | |
 2-methylfuran | dichloromethane | N Parameter: 3.61 sN Parameter: 1.11 | J. Am. Chem. Soc. 2001, 123, 9500-9512 DOI: 10.1021/ja010890y | |
 2-methyl-pent-1-ene | dichloromethane | N Parameter: 0.84 sN Parameter: 1.06 | J. Am. Chem. Soc. 2012, 134, 13902-13911 DOI: 10.1021/ja306522b | |
 2-methyl-buta-1,3-diene (isoprene) | dichloromethane | N Parameter: 1.10 sN Parameter: 0.98 | J. Am. Chem. Soc. 2001, 123, 9500-9512 DOI: 10.1021/ja010890y | |
 2-methyl-but-2-ene | dichloromethane | N Parameter: 0.65 sN Parameter: 1.17 | J. Am. Chem. Soc. 2001, 123, 9500-9512 DOI: 10.1021/ja010890y | |
 2-methyl-but-1-ene | dichloromethane | N Parameter: 1.00 sN Parameter: 1.00 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
 2-methyl-1-(trimethylsiloxy)propen | dichloromethane | N Parameter: 3.94 sN Parameter: 1.00 | J. Am. Chem. Soc. 2001, 123, 9500-9512 DOI: 10.1021/ja010890y | |
 2-methoxy-2H-benzo[d][1,3]dithiol-2-ide 1,1,3,3-tetraoxide (in DMSO) | DMSO | N Parameter: 17.36 sN Parameter: 0.71 | Angew. Chem. Int. Ed. 2016, 55, 12845-12848 DOI: 10.1002/anie.201605616 | |
 2-fluoro-2H-benzo[d][1,3]dithiol-2-ide 1,1,3,3-tetraoxide (in DMSO) | DMSO | N Parameter: 19.03 sN Parameter: 0.58 | Angew. Chem. Int. Ed. 2016, 55, 12845-12848 DOI: 10.1002/anie.201605616 | |
 2-ethoxy-2-oxo-1-(pyridin-1-ium-1-yl)ethan-1-ide (in DMSO) | DMSO | N Parameter: 26.71 sN Parameter: 0.37 | J. Am. Chem. Soc. 2013, 135, 15216-15224 DOI: 10.1021/ja407885h | |
 2-ethoxy-2-oxo-1-(phenylsulfonyl)ethan-1-ide (in DMSO) | DMSO | N Parameter: 18.81 sN Parameter: 0.59 | Angew. Chem. Int. Ed. 2016, 55, 12845-12848 DOI: 10.1002/anie.201605616 | |
 2-ethoxy-1-methoxy-2-oxo-1-(phenylsulfonyl)ethan-1-ide (in DMSO) | DMSO | N Parameter: 19.15 sN Parameter: 0.59 | Angew. Chem. Int. Ed. 2016, 55, 12845-12848 DOI: 10.1002/anie.201605616 | |
 2-ethoxy-1-fluoro-2-oxo-1-(phenylsulfonyl)ethan-1-ide (in DMSO) | DMSO | N Parameter: 20.51 sN Parameter: 0.64 | Angew. Chem. Int. Ed. 2016, 55, 12845-12848 DOI: 10.1002/anie.201605616 | |
 2-diazoindandione | dichloromethane | N Parameter: 0.16 sN Parameter: 0.86 | Eur. J. Org. Chem. 2023, 26, e202300005 DOI: 10.1002/ejoc.202300005 | |
 2-diazoindan-1-one | dichloromethane | N Parameter: 5.61 sN Parameter: 0.65 | Eur. J. Org. Chem. 2023, 26, e202300005 DOI: 10.1002/ejoc.202300005 | |
 2-diazocyclohexanone | dichloromethane | N Parameter: 3.44 sN Parameter: 0.83 | Eur. J. Org. Chem. 2023, 26, e202300005 DOI: 10.1002/ejoc.202300005 | |
 2-diazobenzothiophen-3(2H)-one | dichloromethane | N Parameter: 0.40 sN Parameter: 0.93 | Eur. J. Org. Chem. 2023, 26, e202300005 DOI: 10.1002/ejoc.202300005 | |
 2-diazo-1-tetralone | dichloromethane | N Parameter: 3.51 sN Parameter: 0.86 | Eur. J. Org. Chem. 2023, 26, e202300005 DOI: 10.1002/ejoc.202300005 | |
 2-diazo-1-benzosuberone | dichloromethane | N Parameter: 2.72 sN Parameter: 0.96 | Eur. J. Org. Chem. 2023, 26, e202300005 DOI: 10.1002/ejoc.202300005 | |
 2-cyclopropylpropene | dichloromethane | N Parameter: 2.60 sN Parameter: 1.00 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
 2-chloropropene | dichloromethane | N Parameter: -3.65 sN Parameter: 1.97 | J. Am. Chem. Soc. 2012, 134, 13902-13911 DOI: 10.1021/ja306522b | |
 2-chloro-1,3-diethoxy-1,3-dioxopropan-2-ide (in DMSO) | DMSO | N Parameter: 18.19 sN Parameter: 0.74 | Angew. Chem. Int. Ed. 2016, 55, 12845-12848 DOI: 10.1002/anie.201605616 | |
 2-(tris(trimethylsilyl)silyl)propene | dichloromethane | N Parameter: -0.31 sN Parameter: 0.99 | Chem. Eur. J. 2014, 20, 1103-1110 DOI: 10.1002/chem.201303215 | |
 2-(tris(trimethylsilyl)siloxy)propene | dichloromethane | N Parameter: 6.04 sN Parameter: 0.82 | Org. Lett. 2010, 12, 5206-5209 DOI: 10.1021/ol102220e | |
 2-(tris(pentafluorophenyl)siloxy)-propene | dichloromethane | N Parameter: 0.58 sN Parameter: 0.91 | Eur. J. Org. Chem. 2005, , 1760-1764 DOI: 10.1002/ejoc.200400706 | |
 2-(triphenylsiloxy)propene | dichloromethane | N Parameter: 4.46 sN Parameter: 0.90 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
 2-(trimethylsilyl)thiophene | dichloromethane | N Parameter: -0.80 sN Parameter: 1.00 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
 2-(trimethylsilyl)propene | dichloromethane | N Parameter: -1.46 sN Parameter: 1.05 | Chem. Eur. J. 2014, 20, 1103-1110 DOI: 10.1002/chem.201303215 | |
 2-(trimethylsilyl)furan | dichloromethane | N Parameter: 2.16 sN Parameter: 1.10 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
 2-(trimethylsiloxy)propene | dichloromethane | N Parameter: 5.41 sN Parameter: 0.91 | J. Am. Chem. Soc. 2001, 123, 9500-9512 DOI: 10.1021/ja010890y | |
 2-(trimethylsiloxy)furan | dichloromethane | N Parameter: 7.22 sN Parameter: 1.00 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
 2-(trimethylsiloxy)buta-1,3-diene | dichloromethane | N Parameter: 4.83 sN Parameter: 0.90 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
 2-(trimethylsiloxy)-5,6-dihydro-4H-pyran (in MeCN) | MeCN | N Parameter: 10.52 sN Parameter: 0.78 | J. Am. Chem. Soc. 2013, 135, 6579-6587 DOI: 10.1021/ja401106x | |
 2-(trimethylsiloxy)-5,6-dihydro-4H-pyran | dichloromethane | N Parameter: 10.61 sN Parameter: 0.86 | J. Am. Chem. Soc. 2001, 123, 9500-9512 DOI: 10.1021/ja010890y | |
 2-(triisopropylsiloxy)propene | dichloromethane | N Parameter: 5.38 sN Parameter: 0.85 | J. Am. Chem. Soc. 2001, 123, 9500-9512 DOI: 10.1021/ja010890y | |
 2-(triethylsilyl)furan | dichloromethane | N Parameter: 2.20 sN Parameter: 1.10 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
 2-(tributylstannyl)thiophene | dichloromethane | N Parameter: 1.53 sN Parameter: 1.00 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
 2-(tributylstannyl)furan | dichloromethane | N Parameter: 3.63 sN Parameter: 1.10 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
 2-(tributylsilyl)furan | dichloromethane | N Parameter: 2.37 sN Parameter: 1.10 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
 2-(tert.butyldimethylsiloxy)propene | dichloromethane | N Parameter: 5.58 sN Parameter: 0.90 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
 2-(tert-butoxy)-1-((diphenylmethylene)amino)-2-oxoethan-1-ide (in DMSO) | DMSO | N Parameter: 27.77 sN Parameter: 0.47 | Tetrahedron 2019, 75, 459-463 DOI: 10.1016/j.tet.2018.11.075 | |
 2-(pyrid-4-yl)CH(-)CO2Et (in DMSO) | DMSO | N Parameter: 23.27 sN Parameter: 0.70 | Eur. J. Org. Chem. 2013, , 4255-4261 DOI: 10.1002/ejoc.201300265 | |
 2-(pentamethyldisilyl)propene | dichloromethane | N Parameter: -0.26 sN Parameter: 0.95 | Chem. Eur. J. 2014, 20, 1103-1110 DOI: 10.1002/chem.201303215 | |
 2-(diethylamino)-2-oxo-1-(pyridin-1-ium-1-yl)ethan-1-ide (in DMSO) | DMSO | N Parameter: 27.45 sN Parameter: 0.38 | J. Am. Chem. Soc. 2013, 135, 15216-15224 DOI: 10.1021/ja407885h | |
 2-(bis(trimethylsiloxy)amino)propene | dichloromethane | N Parameter: 4.76 sN Parameter: 0.86 | J. Org. Chem. 2001, 66, 3196-3200 DOI: 10.1021/jo0015927 | |
 2-(bis(tert-butyldimethylsiloxy)amino)propene | dichloromethane | N Parameter: 4.23 sN Parameter: 0.93 | J. Org. Chem. 2001, 66, 3196-3200 DOI: 10.1021/jo0015927 | |
 2-(benzylideneamino)-1-ethoxy-1-oxopropan-2-ide (in DMSO) | DMSO | N Parameter: 30.82 sN Parameter: 0.41 | Tetrahedron 2019, 75, 459-463 DOI: 10.1016/j.tet.2018.11.075 | |
 2-(4-NO2-C6H4)CH(-)CO2Et (in DMSO) | DMSO | N Parameter: 20.00 sN Parameter: 0.71 | Eur. J. Org. Chem. 2013, , 4255-4261 DOI: 10.1002/ejoc.201300265 | |
 2-(4-CN-C6H4)CH(-)CO2Et (in DMSO) | DMSO | N Parameter: 23.64 sN Parameter: 0.65 | Eur. J. Org. Chem. 2013, , 4255-4261 DOI: 10.1002/ejoc.201300265 | |
 2-(4-Br-C6H4)CH(-)CO2Et (in DMSO) | DMSO | N Parameter: 27.62 sN Parameter: 0.53 | Eur. J. Org. Chem. 2013, , 4255-4261 DOI: 10.1002/ejoc.201300265 | |
 2-(3-Cl-C6H4)CH(-)CO2Et (in DMSO) | DMSO | N Parameter: 27.57 sN Parameter: 0.53 | Eur. J. Org. Chem. 2013, , 4255-4261 DOI: 10.1002/ejoc.201300265 | |
 2-((2,2,5,5-tetramethyl-1-styryl-2,5-dihydro-1H-imidazol-4-yl)thio)acetonitrile (in MeCN) | MeCN | N Parameter: 7.74 sN Parameter: 0.87 | Chem. Commun. 2023, 59, 8091-8084 DOI: 10.1039/d3cc01912h | |
 2-((2,2,5,5-tetramethyl-1-styryl-2,5-dihydro-1H-imidazol-4-yl)thio)acetonitrile (in CH2Cl2) | dichloromethane | N Parameter: 7.06 sN Parameter: 1.11 | Chem. Commun. 2023, 59, 8091-8084 DOI: 10.1039/d3cc01912h | |
 2-((2,2,5,5-tetramethyl-1-(prop-1-en-1-yl)-2,5-dihydro-1H-imidazol-4-yl)thio)acetonitrile (in MeCN) | MeCN | N Parameter: 9.25 sN Parameter: 0.84 | Chem. Commun. 2023, 59, 8091-8084 DOI: 10.1039/d3cc01912h | |
 2,7-dimethylocta-1,7-diene | dichloromethane | N Parameter: 1.58 sN Parameter: 1.00 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
 2,6-dimethylphenyl isocyanide | dichloromethane | N Parameter: 4.59 sN Parameter: 0.87 | Angew. Chem. Int. Ed. 2007, 46, 3563-3566 DOI: 10.1002/anie.200605205 | |
 2,6-dimethyl-hepta-1,6-diene | dichloromethane | N Parameter: 1.69 sN Parameter: 1.00 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
 2,5-dimethylpyrrole | MeCN | N Parameter: 8.01 sN Parameter: 0.96 | Eur. J. Org. Chem. 2008, , 2369-2374 DOI: 10.1002/ejoc.200800092 | |
 2,5-dimethylindole | MeCN | N Parameter: 7.22 sN Parameter: 1.10 | J. Org. Chem. 2006, 71, 9088-9095 DOI: 10.1021/jo0614339 | |
 2,5-dimethyl-hexa-1,5-diene | dichloromethane | N Parameter: 1.14 sN Parameter: 1.00 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
 2,4-dimethylpyrrole | MeCN | N Parameter: 10.67 sN Parameter: 0.91 | Eur. J. Org. Chem. 2008, , 2369-2374 DOI: 10.1002/ejoc.200800092 | |
 2,4-dimethyl-penta-1,4-diene | dichloromethane | N Parameter: 0.54 sN Parameter: 1.00 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
 2,4,6-trimethylstyrene | dichloromethane | N Parameter: 0.68 sN Parameter: 1.09 | Macromolecules 2005, 38, 33-40 DOI: 10.1021/ma048389o | |
 2,4,4-trimethyl-pent-1-ene | dichloromethane | N Parameter: 0.79 sN Parameter: 1.07 | J. Am. Chem. Soc. 2012, 134, 13902-13911 DOI: 10.1021/ja306522b | |
 2,3-dimethyl-buta-1,3-diene | dichloromethane | N Parameter: 1.17 sN Parameter: 1.00 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
 2,3-dimethyl-but-1-ene | dichloromethane | N Parameter: 0.65 sN Parameter: 1.00 | J. Am. Chem. Soc. 2012, 134, 13902-13911 DOI: 10.1021/ja306522b | |
 2,3-dihydrofuran | dichloromethane | N Parameter: 4.37 sN Parameter: 0.90 | Eur. J. Org. Chem. 2005, , 1760-1764 DOI: 10.1002/ejoc.200400706 | |
 2,3,3-trimethyl-but-1-ene | dichloromethane | N Parameter: 0.06 sN Parameter: 1.07 | J. Am. Chem. Soc. 2012, 134, 13902-13911 DOI: 10.1021/ja306522b | |
 2,2-dimethyl-5-(4-nitrobenzyl)-4,6-dioxo-1,3-dioxan-5-ide (in DMSO) | DMSO | N Parameter: 12.02 sN Parameter: 1.17 | Chem. Eur. J. 2014, 20, 11069-11077 DOI: 10.1002/chem.201403161 | |
 1H-indole-5-carboxylic acid | MeCN | N Parameter: 3.97 sN Parameter: 1.10 | J. Org. Chem. 2006, 71, 9088-9095 DOI: 10.1021/jo0614339 | |
 1-pyrrolidino-3,4-dihydronaphthalene (in MeCN) | MeCN | N Parameter: 14.09 sN Parameter: 0.66 | Synthesis 2019, 51, 1157-1170 DOI: 10.1055/s-0037-1611634 | |
 1-phenyl-1-(trimethylsiloxy)ethene | dichloromethane | N Parameter: 6.22 sN Parameter: 0.96 | J. Am. Chem. Soc. 2001, 123, 9500-9512 DOI: 10.1021/ja010890y | |
 1-phenoxy-1-(trimethylsiloxy)ethene | dichloromethane | N Parameter: 8.23 sN Parameter: 0.81 | J. Am. Chem. Soc. 2001, 123, 9500-9512 DOI: 10.1021/ja010890y | |
 1-methylcyclopentene | dichloromethane | N Parameter: 1.18 sN Parameter: 1.17 | J. Am. Chem. Soc. 2012, 134, 13902-13911 DOI: 10.1021/ja306522b | |
 1-methylcyclohexene | dichloromethane | N Parameter: 0.08 sN Parameter: 1.15 | J. Am. Chem. Soc. 2001, 123, 9500-9512 DOI: 10.1021/ja010890y | |
 1-methyl-4-vinyl-benzene | dichloromethane | N Parameter: 1.70 sN Parameter: 1.06 | J. Am. Chem. Soc. 2001, 123, 9500-9512 DOI: 10.1021/ja010890y | |
 1-methyl-4-(N-morpholino)tetrahydropyridine | dichloromethane | N Parameter: 12.03 sN Parameter: 0.80 | Chem. Eur. J. 2003, 9, 2209-2218 DOI: 10.1002/chem.200204666 | |
 1-methyl-2-methylene-3-phenyl-1,2-dihydropyridine (in DMSO) | DMSO | N Parameter: 19.41 sN Parameter: 0.61 | Eur. J. Org. Chem. 2024, 27, e202400373 DOI: 10.1002/ejoc.202400373 | |
 1-methoxy-2-methyl-1-(trimethylsiloxy)propene (in MeCN) | MeCN | N Parameter: 9.11 sN Parameter: 0.88 | J. Am. Chem. Soc. 2013, 135, 6579-6587 DOI: 10.1021/ja401106x | |
 1-methoxy-2-methyl-1-(trimethylsiloxy)propene | dichloromethane | N Parameter: 9.00 sN Parameter: 0.98 | J. Am. Chem. Soc. 2001, 123, 9500-9512 DOI: 10.1021/ja010890y | |
 1-mesityl-3-methyl-5-methylene-2,4,6-triphenyl-2,5-dihydropyridin-1-ium-2-ide (in THF) | THF | N Parameter: 26.16 sN Parameter: 0.52 | Angew. Chem. Int. Ed. 2024, , e202318283 DOI: 10.1002/anie.202318283 | |
 1-hexene | dichloromethane | N Parameter: -2.77 sN Parameter: 1.41 | J. Am. Chem. Soc. 2012, 134, 13902-13911 DOI: 10.1021/ja306522b | |
 1-ethoxy-3-(4-nitrophenyl)-1,3-dioxopropan-2-ide (in DMSO) | DMSO | N Parameter: 16.26 sN Parameter: 0.83 | J. Am. Chem. Soc. 2018, 140, 11474-11486 DOI: 10.1021/jacs.8b07147 | |
 1-ethoxy-2-methyl-1,3-dioxobutan-2-ide (in DMSO) | DMSO | N Parameter: 19.58 sN Parameter: 0.73 | Eur. J. Org. Chem. 2015, , 7594-7601 DOI: 10.1002/ejoc.201501107 | |
 1-ethinylcyclohexenyl-Co2(CO)6 | dichloromethane | N Parameter: -0.44 sN Parameter: 1.06 | J. Am. Chem. Soc. 2001, 123, 9500-9512 DOI: 10.1021/ja010890y | |
 1-butoxy-1-(trimethylsiloxy)ethene | dichloromethane | N Parameter: 10.21 sN Parameter: 0.82 | Eur. J. Org. Chem. 2004, , 2791-2796 DOI: 10.1002/ejoc.200400134 | |
 1-butoxy-1-(t-butyl-dimethylsiloxy)ethene | dichloromethane | N Parameter: 10.32 sN Parameter: 0.79 | Eur. J. Org. Chem. 2004, , 2791-2796 DOI: 10.1002/ejoc.200400134 | |
 1-(tris(trimethylsilyl)siloxy)cyclohexene | dichloromethane | N Parameter: 5.07 sN Parameter: 0.91 | Org. Lett. 2010, 12, 5206-5209 DOI: 10.1021/ol102220e | |
 1-(tris(pentafluorophenyl)siloxy)-cyclopentene | dichloromethane | N Parameter: 1.38 sN Parameter: 0.93 | Eur. J. Org. Chem. 2005, , 1760-1764 DOI: 10.1002/ejoc.200400706 | |
 1-(triphenylsiloxy)cyclopentene | dichloromethane | N Parameter: 5.76 sN Parameter: 1.02 | Eur. J. Org. Chem. 2005, , 1760-1764 DOI: 10.1002/ejoc.200400706 | |
 1-(trimethylsiloxy)cyclopentene (in MeCN) | MeCN | N Parameter: 6.43 sN Parameter: 0.89 | Chem. Asian J. 2012, 7, 1401-1407 DOI: 10.1002/asia.201101046 | |
 1-(trimethylsiloxy)cyclopentene | dichloromethane | N Parameter: 6.57 sN Parameter: 0.93 | J. Am. Chem. Soc. 2001, 123, 9500-9512 DOI: 10.1021/ja010890y | |
 1-(trimethylsiloxy)cyclooctene | dichloromethane | N Parameter: 6.77 sN Parameter: 1.00 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
 1-(trimethylsiloxy)cyclohexene | dichloromethane | N Parameter: 5.21 sN Parameter: 1.00 | J. Am. Chem. Soc. 2001, 123, 9500-9512 DOI: 10.1021/ja010890y | |
 1-(trimethylsiloxy)cycloheptene | dichloromethane | N Parameter: 6.62 sN Parameter: 1.00 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
 1-(trimethylsiloxy)buta-1,3-diene | dichloromethane | N Parameter: 4.60 sN Parameter: 0.90 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
 1-(trimethylsiloxy)-3,4-dihydronaphthalene (in MeCN) | MeCN | N Parameter: 5.06 sN Parameter: 0.91 | Synthesis 2019, 51, 1157-1170 DOI: 10.1055/s-0037-1611634 | |
 1-(phenylmethylamino)cyclopentene | dichloromethane | N Parameter: 12.90 sN Parameter: 0.79 | Chem. Eur. J. 2003, 9, 2209-2218 DOI: 10.1002/chem.200204666 | |
 1-(phenylmethylamino)cyclohexene | dichloromethane | N Parameter: 10.73 sN Parameter: 0.81 | Chem. Eur. J. 2003, 9, 2209-2218 DOI: 10.1002/chem.200204666 | |
 1-(phenylethynyl)pyrrolidin-2-one | dichloromethane | N Parameter: 3.12 sN Parameter: 0.85 | Angew. Chem. Int. Ed. 2014, 53, 4968-4971 DOI: 10.1002/anie.201402055 | |
 1-(perfluorophenyl)-1-(trimethylsiloxy)ethene | dichloromethane | N Parameter: 1.47 sN Parameter: 0.89 | Org. Lett. 2012, 14, 3990-3993 DOI: 10.1021/ol301766w | |
 1-(p-tolyl)-1-(trimethylsilyl)ethene | dichloromethane | N Parameter: -0.65 sN Parameter: 1.59 | Chem. Eur. J. 2014, 20, 1103-1110 DOI: 10.1002/chem.201303215 | |
 1-(N-pyrrolidino)cyclopentene | dichloromethane | N Parameter: 15.91 sN Parameter: 0.86 | Chem. Eur. J. 2003, 9, 2209-2218 DOI: 10.1002/chem.200204666 | |
 1-(N-pyrrolidino)cyclohexene (in MeCN) | MeCN | N Parameter: 16.42 sN Parameter: 0.70 | Angew. Chem. Int. Ed. 2010, 49, 9526-9529 DOI: 10.1002/anie.201004344 | |
 1-(N-pyrrolidino)cyclohexene | dichloromethane | N Parameter: 14.91 sN Parameter: 0.86 | Chem. Eur. J. 2003, 9, 2209-2218 DOI: 10.1002/chem.200204666 | |
 1-(N-piperidino)cyclopentene | dichloromethane | N Parameter: 15.06 sN Parameter: 0.82 | Chem. Eur. J. 2003, 9, 2209-2218 DOI: 10.1002/chem.200204666 | |
 1-(N-piperidino)cyclohexene (in MeCN) | MeCN | N Parameter: 14.02 sN Parameter: 0.76 | J. Am. Chem. Soc. 2013, 135, 6579-6587 DOI: 10.1021/ja401106x | |
 1-(N-piperidino)cyclohexene | dichloromethane | N Parameter: 13.36 sN Parameter: 0.81 | J. Am. Chem. Soc. 2001, 123, 9500-9512 DOI: 10.1021/ja010890y | |
 1-(N-morpholino)cyclopentene | dichloromethane | N Parameter: 13.41 sN Parameter: 0.82 | Chem. Eur. J. 2003, 9, 2209-2218 DOI: 10.1002/chem.200204666 | |
 1-(N-morpholino)cyclohexene | dichloromethane | N Parameter: 11.40 sN Parameter: 0.83 | J. Am. Chem. Soc. 2001, 123, 9500-9512 DOI: 10.1021/ja010890y | |
 1-(isoquinolin-2-ium-2-yl)-2-oxo-2-phenylethan-1-ide (in DMSO) | DMSO | N Parameter: 20.08 sN Parameter: 0.57 | J. Am. Chem. Soc. 2013, 135, 15216-15224 DOI: 10.1021/ja407885h | |
 1-(ethoxycarbonyl)-2-oxocyclopentan-1-ide (in DMSO) | DMSO | N Parameter: 18.63 sN Parameter: 0.82 | Eur. J. Org. Chem. 2015, , 7594-7601 DOI: 10.1002/ejoc.201501107 | |
 1-(ethoxycarbonyl)-2-oxocyclooctan-1-ide (in DMSO) | DMSO | N Parameter: 20.02 sN Parameter: 0.76 | Eur. J. Org. Chem. 2015, , 7594-7601 DOI: 10.1002/ejoc.201501107 | |
 1-(ethoxycarbonyl)-2-oxocyclohexan-1-ide (in DMSO) | DMSO | N Parameter: 18.32 sN Parameter: 0.84 | Eur. J. Org. Chem. 2015, , 7594-7601 DOI: 10.1002/ejoc.201501107 | |
 1-(ethoxycarbonyl)-2-oxocycloheptan-1-ide | DMSO | N Parameter: 19.53 sN Parameter: 0.83 | Eur. J. Org. Chem. 2015, , 7594-7601 DOI: 10.1002/ejoc.201501107 | |
 1-(ethoxycarbonyl)-2-oxocyclododecan-1-ide (in DMSO) | DMSO | N Parameter: 20.60 sN Parameter: 0.63 | Eur. J. Org. Chem. 2015, , 7594-7601 DOI: 10.1002/ejoc.201501107 | |
 1-(benzylideneamino)-2-ethoxy-2-oxoethan-1-ide (in DMSO) | DMSO | N Parameter: 29.10 sN Parameter: 0.50 | Tetrahedron 2019, 75, 459-463 DOI: 10.1016/j.tet.2018.11.075 | |
 1-(4-(tert-butyl)phenyl)-2-methylene-4,6-diphenyl-1,2-dihydropyridine (in DMSO) | DMSO | N Parameter: 19.36 sN Parameter: 0.68 | Eur. J. Org. Chem. 2024, 27, e202400373 DOI: 10.1002/ejoc.202400373 | |
 1-(4-(dimethylamino)pyridin-1-ium-1-yl)-2-oxo-2-phenylethan-1-ide (in DMSO) | DMSO | N Parameter: 21.61 sN Parameter: 0.58 | J. Am. Chem. Soc. 2013, 135, 15216-15224 DOI: 10.1021/ja407885h | |
 1-(3-chloropyridin-1-ium-1-yl)-2-oxo-2-phenylethan-1-ide (in DMSO) | DMSO | N Parameter: 17.98 sN Parameter: 0.63 | J. Am. Chem. Soc. 2013, 135, 15216-15224 DOI: 10.1021/ja407885h | |
 1-(1-cyclohexenyl)-4-methylpiperazine | dichloromethane | N Parameter: 12.51 sN Parameter: 0.80 | Chem. Eur. J. 2003, 9, 2209-2218 DOI: 10.1002/chem.200204666 | |
 1-((diphenylmethylene)amino)-2-ethoxy-2-oxoethan-1-ide (in DMSO) | DMSO | N Parameter: 26.95 sN Parameter: 0.52 | Tetrahedron 2019, 75, 459-463 DOI: 10.1016/j.tet.2018.11.075 | |
 1-((4-chlorobenzylidene)amino)-2-ethoxy-2-oxoethan-1-ide (in DMSO) | DMSO | N Parameter: 29.02 sN Parameter: 0.49 | Tetrahedron 2019, 75, 459-463 DOI: 10.1016/j.tet.2018.11.075 | |
 1,6-dimethyl-2-methylene-1,2-dihydropyridine (in DMSO) | DMSO | N Parameter: 20.76 sN Parameter: 0.60 | Eur. J. Org. Chem. 2024, 27, e202400373 DOI: 10.1002/ejoc.202400373 | |
 1,3-dimethylbarbiturate anion (in DMSO) | DMSO | N Parameter: 17.46 sN Parameter: 0.72 | J. Org. Chem. 2017, 82, 8476-8488 DOI: 10.1021/acs.joc.7b01223 | |
 1,3-dimethyl-2-methyleneimidazolidine (in THF) | THF | N Parameter: 18.11 sN Parameter: 0.68 | J. Org. Chem. 2021, 86, 2974-2985 DOI: 10.1021/acs.joc.0c02838 | |
 1,3-dimethyl-2-methylenehexahydropyrimidine (in THF) | THF | N Parameter: 18.68 sN Parameter: 0.63 | J. Org. Chem. 2021, 86, 2974-2985 DOI: 10.1021/acs.joc.0c02838 | |
 1,3-dimethyl-2-methylene-2,3-dihydro-1H-benzo[d]imidazole | THF | N Parameter: 19.84 sN Parameter: 0.58 | J. Org. Chem. 2021, 86, 2974-2985 DOI: 10.1021/acs.joc.0c02838 | |
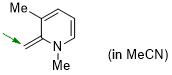 1,3-dimethyl-2-methylene-1,2-dihydropyridine (in MeCN) | MeCN | N Parameter: 19.69 sN Parameter: 0.61 | Eur. J. Org. Chem. 2024, 27, e202400373 DOI: 10.1002/ejoc.202400373 | |
 1,3-dimethyl-2-methylene-1,2-dihydropyridine (in DMSO) | DMSO | N Parameter: 19.91 sN Parameter: 0.62 | Eur. J. Org. Chem. 2024, 27, e202400373 DOI: 10.1002/ejoc.202400373 | |
 1,3-dimethoxybenzene | dichloromethane | N Parameter: 2.48 sN Parameter: 1.09 | J. Am. Chem. Soc. 2001, 123, 9500-9512 DOI: 10.1021/ja010890y | |
 1,3-dimesityl-4,5-dihydro-1H-imidazol-3-ium-2-ide (in THF) | THF | N Parameter: 23.35 sN Parameter: 0.40 | Angew. Chem. Int. Ed. 2011, 50, 6915-6919 DOI: 10.1002/anie.201102435 | |
 1,3-dimesityl-2-methylene-2,3-dihydro-1H-imidazole (in THF) | THF | N Parameter: 17.80 sN Parameter: 0.79 | J. Org. Chem. 2021, 86, 2974-2985 DOI: 10.1021/acs.joc.0c02838 | |
 1,3-dimesityl-1H-imidazol-3-ium-2-ide (in THF) | THF | N Parameter: 21.72 sN Parameter: 0.45 | Angew. Chem. Int. Ed. 2011, 50, 6915-6919 DOI: 10.1002/anie.201102435 | |
 1,3-Diisopropyl-4-methylene-5-phenyl-4,5-dihydro-1H-1,2,3-triazol-3-ium-5-ide (in THF) | THF | N Parameter: 30.25 sN Parameter: 0.54 | Angew. Chem. Int. Ed. 2023, 62, e202309790 DOI: 10.1002/anie.202309790 | |
 1,3-diethyl-thiobarbiturate anion (in DMSO) | DMSO | N Parameter: 14.90 sN Parameter: 0.80 | J. Org. Chem. 2017, 82, 8476-8488 DOI: 10.1021/acs.joc.7b01223 | |
 1,3-diethoxy-2-fluoro-1,3-dioxopropan-2-ide (in DMSO) | DMSO | N Parameter: 20.63 sN Parameter: 0.76 | Angew. Chem. Int. Ed. 2016, 55, 12845-12848 DOI: 10.1002/anie.201605616 | |
 1,3-Bis(2,6-diisopropylphenyl)-5-methylene-4-(2,4,6-triisopropylphenyl)-4,5-dihydro-1H-1,2,3-triazol-3-ium-4-ide (in THF) | THF | N Parameter: 21.36 sN Parameter: 0.42 | Angew. Chem. Int. Ed. 2023, 62, e202309790 DOI: 10.1002/anie.202309790 | |
 1,3-Bis(2,6-diisopropylphenyl)-5-methylene-4-(2,4,6-triisopropylphenyl)-4,5-dihydro-1H-1,2,3-triazol-3-ium-4-ide (in THF) | THF | N Parameter: 20.78 sN Parameter: 0.61 | Angew. Chem. Int. Ed. 2023, 62, e202309790 DOI: 10.1002/anie.202309790 | |
 1,3-bis(2,6-diisopropylphenyl)-5-methyl-4-methylene-4,5-dihydro-1H-1,2,3-triazol-3-ium-5-ide (in THF) | THF | N Parameter: 24.86 sN Parameter: 0.52 | Angew. Chem. Int. Ed. 2023, 62, e202309790 DOI: 10.1002/anie.202309790 | |
 1,3-Bis(2,6-diisopropylphenyl)-5-(4-methoxyphenyl)-4-methylene-4,5-dihydro-1H-1,2,3-triazol-3-ium-5-ide (in THF) | THF | N Parameter: 23.55 sN Parameter: 0.56 | Angew. Chem. Int. Ed. 2023, 62, e202309790 DOI: 10.1002/anie.202309790 | |
 1,3-bis(2,6-diisopropylphenyl)-4-methylene-5-phenyl-4,5-dihydro-1H-1,2,3-triazol-3-ium-5-ide (in THF) | THF | N Parameter: 22.80 sN Parameter: 0.60 | Angew. Chem. Int. Ed. 2023, 62, e202309790 DOI: 10.1002/anie.202309790 | |
 1,3-Bis(2,6-diisopropylphenyl)-4-methylene-5-(4-(trifluoromethyl)phenyl)-4,5-dihydro-1H-1,2,3-triazol-3-ium-5-ide (in THF) | THF | N Parameter: 22.30 sN Parameter: 0.49 | Angew. Chem. Int. Ed. 2023, 62, e202309790 DOI: 10.1002/anie.202309790 | |
 1,3,5-trimethyl-2-methylene-1,2-dihydropyridine (in DMSO) | DMSO | N Parameter: 21.16 sN Parameter: 0.59 | Eur. J. Org. Chem. 2024, 27, e202400373 DOI: 10.1002/ejoc.202400373 | |
 1,3,4-triphenyl-1H-1,2,4-triazol-4-ium-5-ide (in THF) | THF | N Parameter: 14.07 sN Parameter: 0.84 | Angew. Chem. Int. Ed. 2011, 50, 6915-6919 DOI: 10.1002/anie.201102435 | |
 1,2-dimethylindole | dichloromethane | N Parameter: 6.54 sN Parameter: 1.10 | J. Org. Chem. 2006, 71, 9088-9095 DOI: 10.1021/jo0614339 | |
 1,2-bis(trimethylsiloxy)cyclohexene | dichloromethane | N Parameter: 4.46 sN Parameter: 1.15 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
 1,2,5-trimethylpyrrole | MeCN | N Parameter: 8.69 sN Parameter: 1.07 | Eur. J. Org. Chem. 2008, , 2369-2374 DOI: 10.1002/ejoc.200800092 | |
 1,2,3-trimethoxy-1,3-dioxopropan-2-ide (in DMSO) | DMSO | N Parameter: 20.08 sN Parameter: 0.74 | Angew. Chem. Int. Ed. 2016, 55, 12845-12848 DOI: 10.1002/anie.201605616 | |
 1,1-dimethyl-2-methylenehydrazine (in CH2Cl2) | dichloromethane | N Parameter: 6.98 sN Parameter: 0.85 | Angew. Chem. Int. Ed. 2013, 52, 11900-11904 DOI: 10.1002/anie.201305092 | |
 1,1-diethoxyethene | dichloromethane | N Parameter: 9.81 sN Parameter: 0.81 | Eur. J. Org. Chem. 2004, , 2791-2796 DOI: 10.1002/ejoc.200400134 | |
 1,1-bis(trimethylsiloxy)propene | dichloromethane | N Parameter: 10.38 sN Parameter: 0.87 | Eur. J. Org. Chem. 2004, , 2791-2796 DOI: 10.1002/ejoc.200400134 | |
 (Z)-phenylpropene | dichloromethane | N Parameter: -1.07 sN Parameter: 1.10 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
 (Z)-penta-1,3-diene | dichloromethane | N Parameter: 0.66 sN Parameter: 1.00 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
 (Z)-but-2-enyltrimethylsilane | dichloromethane | N Parameter: 1.69 sN Parameter: 1.10 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
 (Z)-but-2-ene | dichloromethane | N Parameter: -2.44 sN Parameter: 1.09 | J. Am. Chem. Soc. 2001, 123, 9500-9512 DOI: 10.1021/ja010890y | |
 (Z)-2-phenyl-1-(1,3,4-triphenyl-4H-1,2,4-triazol-1-ium-5-yl)prop-1-en-1-olate (in THF) | THF | N Parameter: 14.40 sN Parameter: 0.66 | Angew. Chem. Int. Ed. 2013, 52, 11163-11167 DOI: 10.1002/ange.201303524 | |
 (Z)-1-phenyl-1-(trimethylsiloxy)propene (in MeCN) | MeCN | N Parameter: 5.18 sN Parameter: 0.94 | Synthesis 2019, 51, 1157-1170 DOI: 10.1055/s-0037-1611634 | |
 (Z)-1-(triisopropylsiloxy)propen | dichloromethane | N Parameter: 3.87 sN Parameter: 1.00 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
 (Z)-1-(N-morpholino)propene | dichloromethane | N Parameter: 12.26 sN Parameter: 0.80 | Chem. Eur. J. 2003, 9, 2209-2218 DOI: 10.1002/chem.200204666 | |
 (Z)-1-(1,3-dimesityl-4,5-dihydro-1H-imidazol-3-ium-2-yl)-2-phenylprop-1-en-1-olate (in THF) | THF | N Parameter: 15.92 sN Parameter: 0.72 | Angew. Chem. Int. Ed. 2013, 52, 11163-11167 DOI: 10.1002/ange.201303524 | |
 (Z)-1-(1,3-dimesityl-1H-imidazol-3-ium-2-yl)-2-phenylprop-1-en-1-olate (in THF) | THF | N Parameter: 15.33 sN Parameter: 0.79 | Angew. Chem. Int. Ed. 2013, 52, 11163-11167 DOI: 10.1002/ange.201303524 | |
 (Z)-1-(1,3-dimesityl-1H-imidazol-3-ium-2-yl)-2-phenylprop-1-en-1-olate (in DMSO) | DMSO | N Parameter: 14.40 sN Parameter: 0.64 | Angew. Chem. Int. Ed. 2013, 52, 11163-11167 DOI: 10.1002/ange.201303524 | |
 (Z)-1,2-diphenyl-1-(trimethylsiloxy)ethene (in MeCN) | MeCN | N Parameter: 3.00 sN Parameter: 0.83 | Synthesis 2019, 51, 1157-1170 DOI: 10.1055/s-0037-1611634 | |
 (tris(trimethylsilyl)siloxy)ethene | dichloromethane | N Parameter: 4.01 sN Parameter: 0.83 | Org. Lett. 2010, 12, 5206-5209 DOI: 10.1021/ol102220e | |
 (trimethylsilyl)diazomethane | dichloromethane | N Parameter: 8.97 sN Parameter: 0.75 | Chem. Eur. J. 2003, 9, 4068-4076 DOI: 10.1002/chem.200304913 | |
 (triisopropylsiloxy)ethene | dichloromethane | N Parameter: 3.44 sN Parameter: 0.94 | J. Am. Chem. Soc. 2001, 123, 9500-9512 DOI: 10.1021/ja010890y | |
 (S,E)-5-benzyl-2,2,3-trimethyl-1-styrylimidazolidin-4-one | MeCN | N Parameter: 7.20 sN Parameter: 1.14 | Angew. Chem. Int. Ed. 2012, 51, 5739-5742 DOI: 10.1002/anie.201201240 | |
 (S,E)-3-benzyl-1-methyl-4-styryl-1,4-diazaspiro[4.4]nonan-2-one | MeCN | N Parameter: 7.92 sN Parameter: 1.07 | Angew. Chem. Int. Ed. 2012, 51, 5739-5742 DOI: 10.1002/anie.201201240 | |
 (S,E)-2-(diphenyl(trimethylsiloxy)methyl)-1-styrylpyrrolidine | MeCN | N Parameter: 10.56 sN Parameter: 1.01 | Angew. Chem. Int. Ed. 2012, 51, 5739-5742 DOI: 10.1002/anie.201201240 | |
 (p-nitrophenyl)diazomethane | dichloromethane | N Parameter: 7.17 sN Parameter: 0.83 | J. Am. Chem. Soc. 2018, 140, 16758-16772 DOI: 10.1021/jacs.8b09995 | |
 (p-cyanophenyl)diazomethane | dichloromethane | N Parameter: 7.66 sN Parameter: 0.80 | J. Am. Chem. Soc. 2018, 140, 16758-16772 DOI: 10.1021/jacs.8b09995 | |
 (p-bromophenyl)diazomethane | dichloromethane | N Parameter: 8.87 sN Parameter: 0.82 | J. Am. Chem. Soc. 2018, 140, 16758-16772 DOI: 10.1021/jacs.8b09995 | |
 (EtO)2P(O)CH(-)CO2Et (in DMSO) | DMSO | N Parameter: 19.23 sN Parameter: 0.65 | J. Am. Chem. Soc. 2009, 131, 704-714 DOI: 10.1021/ja8056216 | |
 (EtO)2P(O)CH(-)CN (in DMSO) | DMSO | N Parameter: 18.57 sN Parameter: 0.66 | J. Am. Chem. Soc. 2009, 131, 704-714 DOI: 10.1021/ja8056216 | |
 (E,E)-hexa-2,4-diene | dichloromethane | N Parameter: 1.17 sN Parameter: 1.10 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
 (E)-propenylbenzene | dichloromethane | N Parameter: -0.49 sN Parameter: 1.18 | J. Am. Chem. Soc. 2012, 134, 13902-13911 DOI: 10.1021/ja306522b | |
 (E)-penta-1,3-diene | dichloromethane | N Parameter: 1.49 sN Parameter: 1.00 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
 (E)-but-2-enyltrimethylsilane | dichloromethane | N Parameter: 1.94 sN Parameter: 1.10 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
 (E)-but-2-ene | dichloromethane | N Parameter: -2.45 sN Parameter: 1.10 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
 (E)-beta-(N-piperidino)styrene (in MeCN) | MeCN | N Parameter: 13.84 sN Parameter: 0.73 | Chem. Eur. J. 2018, 24, 5901-5910 DOI: 10.1002/chem.201705962 | |
 (E)-beta-(N-morpholino)styrene | dichloromethane | N Parameter: 10.76 sN Parameter: 0.87 | Chem. Eur. J. 2003, 9, 2209-2218 DOI: 10.1002/chem.200204666 | |
 (E)-4-styrylmorpholine | MeCN | N Parameter: 11.66 sN Parameter: 0.83 | Chem. Eur. J. 2018, 24, 5901-5910 DOI: 10.1002/chem.201705962 | |
 (E)-4-(ethylthio)-2,2,5,5-tetramethyl-1-styryl-2,5-dihydro-1H-imidazole (in MeCN) | MeCN | N Parameter: 8.12 sN Parameter: 0.88 | Chem. Eur. J. 2024, 30, e202302764 DOI: 10.1002/chem.202302764 | |
 (E)-4-(ethylthio)-2,2,5,5-tetramethyl-1-styryl-2,5-dihydro-1H-imidazole (in CH2Cl2) | dichloromethane | N Parameter: 8.13 sN Parameter: 0.97 | Chem. Eur. J. 2024, 30, e202302764 DOI: 10.1002/chem.202302764 | |
 (E)-4-(ethylthio)-2,2,5,5-tetramethyl-1-(3-phenylprop-1-en-1-yl)-2,5-dihydro-1H-imidazole (in MeCN) | MeCN | N Parameter: 9.64 sN Parameter: 0.70 | Chem. Eur. J. 2024, 30, e202302764 DOI: 10.1002/chem.202302764 | |
 (E)-4-(ethylthio)-2,2,5,5-tetramethyl-1-(3-phenylbut-1-en-1-yl)-2,5-dihydro-1H-imidazole (in MeCN) | MeCN | N Parameter: 7.92 sN Parameter: 0.73 | Chem. Eur. J. 2024, 30, e202302764 DOI: 10.1002/chem.202302764 | |
 (E)-4-(benzylthio)-2,2,5,5-tetramethyl-1-styryl-2,5-dihydro-1H-imidazole (in MeCN) | MeCN | N Parameter: 8.30 sN Parameter: 0.84 | Chem. Eur. J. 2024, 30, e202302764 DOI: 10.1002/chem.202302764 | |
 (E)-4-(benzylthio)-2,2,5,5-tetramethyl-1-styryl-2,5-dihydro-1H-imidazole (in CH2Cl2) | dichloromethane | N Parameter: 7.99 sN Parameter: 0.98 | Chem. Eur. J. 2024, 30, e202302764 DOI: 10.1002/chem.202302764 | |
 (E)-4-(2-phenyl-2-(pyrrolidin-1-yl)vinyl)benzonitrile (in MeCN) | MeCN | N Parameter: 10.63 sN Parameter: 0.84 | Chem. Eur. J. 2018, 24, 5901-5910 DOI: 10.1002/chem.201705962 | |
 (E)-4-(1,2-diphenylvinyl)morpholine (in MeCN) | MeCN | N Parameter: 8.78 sN Parameter: 0.83 | Chem. Eur. J. 2018, 24, 5901-5910 DOI: 10.1002/chem.201705962 | |
 (E)-2-methyl-penta-1,3-diene | dichloromethane | N Parameter: 3.09 sN Parameter: 1.00 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
 (E)-2-((1-(but-1-en-1-yl)-2,2,5,5-tetramethyl-2,5-dihydro-1H-imidazol-4-yl)thio)acetonitrile (in MeCN) | MeCN | N Parameter: 9.23 sN Parameter: 0.74 | Chem. Eur. J. 2024, 30, e202302764 DOI: 10.1002/chem.202302764 | |
 (E)-2,2,3,5,5-pentamethyl-1-styrylimidazolidine-4-thione (in MeCN) | MeCN | N Parameter: 6.49 sN Parameter: 0.83 | Chem. Eur. J. 2024, 30, e202302764 DOI: 10.1002/chem.202302764 | |
 (E)-2,2,3,5,5-pentamethyl-1-styrylimidazolidin-4-one (in MeCN) | MeCN | N Parameter: 7.10 sN Parameter: 0.82 | Chem. Eur. J. 2024, 30, e202302764 DOI: 10.1002/chem.202302764 | |
 (E)-13-(ethylthio)-6-styryl-6,12-diazadispiro[4.1.47.25]tridec-12-ene (in MeCN) | MeCN | N Parameter: 7.79 sN Parameter: 0.87 | Chem. Eur. J. 2024, 30, e202302764 DOI: 10.1002/chem.202302764 | |
 (E)-1-styrylpyrrolidine (in MeCN) | MeCN | N Parameter: 13.87 sN Parameter: 0.76 | Chem. Eur. J. 2018, 24, 5901-5910 DOI: 10.1002/chem.201705962 | |
 (E)-1-styrylpyrrolidine (in CH2Cl2) | dichloromethane | N Parameter: 12.26 sN Parameter: 0.93 | J. Am. Chem. Soc. 2014, 136, 14263-14269 DOI: 10.1021/ja508065e | |
 (E)-1-styryl-2-tritylpyrrolidine (in CH2Cl2) | dichloromethane | N Parameter: 10.19 sN Parameter: 1.06 | J. Am. Chem. Soc. 2014, 136, 14263-14269 DOI: 10.1021/ja508065e | |
 (E)-1-styryl-2-(triphenylsilyl)pyrrolidine (in CH2Cl2) | dichloromethane | N Parameter: 11.77 sN Parameter: 0.98 | J. Am. Chem. Soc. 2014, 136, 14263-14269 DOI: 10.1021/ja508065e | |
 (E)-1-(N-morpholino)propene | dichloromethane | N Parameter: 12.06 sN Parameter: 0.80 | Chem. Eur. J. 2003, 9, 2209-2218 DOI: 10.1002/chem.200204666 | |
 (E)-1-(2-(4-nitrophenyl)-1-phenylvinyl)pyrrolidine (in MeCN) | MeCN | N Parameter: 10.42 sN Parameter: 0.82 | Chem. Eur. J. 2018, 24, 5901-5910 DOI: 10.1002/chem.201705962 | |
 (E)-1-(2-(4-methoxyphenyl)-1-phenylvinyl)pyrrolidine (in MeCN) | MeCN | N Parameter: 11.99 sN Parameter: 0.84 | Chem. Eur. J. 2018, 24, 5901-5910 DOI: 10.1002/chem.201705962 | |
 (E)-1-(1,2-diphenylvinyl)pyrrolidine (in MeCN) | MeCN | N Parameter: 11.66 sN Parameter: 0.82 | Chem. Eur. J. 2018, 24, 5901-5910 DOI: 10.1002/chem.201705962 | |
 (E)-1-(1,2-diphenylvinyl)piperidine (in MeCN) | MeCN | N Parameter: 9.94 sN Parameter: 0.86 | Chem. Eur. J. 2018, 24, 5901-5910 DOI: 10.1002/chem.201705962 | |
 (cyclohexen-1-yl)prolinate (in MeCN) | MeCN | N Parameter: 18.86 sN Parameter: 0.70 | Angew. Chem. Int. Ed. 2010, 49, 9526-9529 DOI: 10.1002/anie.201004344 | |
 (Cp*)2Zr(Me)2 - Dimethyl-bis(pentamethylcyclopentadienyl)zirconium | dichloromethane | N Parameter: 5.49 sN Parameter: 1.06 | Chem. Eur. J. 2016, 22, 11196-11200 DOI: 10.1002/chem.201602452 | |
 (C6H5)-(CO)-CH=SMe2 (in DMSO) | DMSO | N Parameter: 13.95 sN Parameter: 0.69 | J. Am. Chem. Soc. 2010, 132, 17894-17900 DOI: 10.1021/ja1084749 | |
 (bis(trimethylsiloxy)amino)styrene | dichloromethane | N Parameter: 4.80 sN Parameter: 0.86 | J. Org. Chem. 2001, 66, 3196-3200 DOI: 10.1021/jo0015927 | |
 (allyl)dicarbonyl(cyclopentadienyl)iron(II) | dichloromethane | N Parameter: 6.78 sN Parameter: 0.95 | Helv. Chim. Acta 2005, 88, 1754-1768 DOI: 10.1002/hlca.200590137 | |
 (5-methyl-furan-2-yl)(1,1,1-tris(methoxy)ethane)borate | MeCN | N Parameter: 12.55 sN Parameter: 0.92 | Chem. Sci. 2012, 3, 878-882 DOI: 10.1039/c2sc00883a | |
 (5-methyl-furan-2-yl) trifluoroborate | MeCN | N Parameter: 7.66 sN Parameter: 1.04 | Chem. Sci. 2012, 3, 878-882 DOI: 10.1039/c2sc00883a | |
 (5-methyl-furan-2-yl) pinacol boronate | MeCN | N Parameter: 2.90 sN Parameter: 0.98 | Chem. Sci. 2012, 3, 878-882 DOI: 10.1039/c2sc00883a | |
 (5-methyl-furan-2-yl) MIDA boronate | MeCN | N Parameter: 1.84 sN Parameter: 1.26 | Chem. Sci. 2012, 3, 878-882 DOI: 10.1039/c2sc00883a | |
 (5-methyl-furan-2-yl) MDA boronate | MeCN | N Parameter: 6.38 sN Parameter: 0.86 | Chem. Sci. 2012, 3, 878-882 DOI: 10.1039/c2sc00883a | |
 (4-MeO-C6H4)-(CO)-CH=SMe2 (in DMSO) | DMSO | N Parameter: 14.48 sN Parameter: 0.71 | J. Am. Chem. Soc. 2010, 132, 17894-17900 DOI: 10.1021/ja1084749 | |
 (4-Me2N-C6H4)-(CO)-CH=SMe2 (in DMSO) | DMSO | N Parameter: 15.68 sN Parameter: 0.65 | J. Am. Chem. Soc. 2010, 132, 17894-17900 DOI: 10.1021/ja1084749 | |
 (4-Br-C6H4)-(CO)-CH=SMe2 (in DMSO) | DMSO | N Parameter: 13.78 sN Parameter: 0.72 | J. Am. Chem. Soc. 2010, 132, 17894-17900 DOI: 10.1021/ja1084749 | |
 (4-Br-C6H4)-(CO)-CH=SMe2 | dichloromethane | N Parameter: 11.95 sN Parameter: 0.76 | Angew. Chem. Int. Ed. 2009, 48, 5034-5037 DOI: 10.1002/anie.200900933 | |
 (3,3,4,4,5,5,6,6,7,7,7-undecafluoro)-2-(trimethylsiloxy)hept-1-ene | dichloromethane | N Parameter: -3.52 sN Parameter: 1.17 | Org. Lett. 2012, 14, 3990-3993 DOI: 10.1021/ol301766w | |
 (2S,5S)-5-benzyl-2-(tert-butyl)-3-methyl-1-((E)-styryl)imidazolidin-4-one | MeCN | N Parameter: 5.80 sN Parameter: 0.87 | Angew. Chem. Int. Ed. 2012, 51, 5739-5742 DOI: 10.1002/anie.201201240 | |
 (2-methylallyl)tris(trimethylsilyl)silane | dichloromethane | N Parameter: 4.63 sN Parameter: 0.87 | Org. Lett. 2010, 12, 5206-5209 DOI: 10.1021/ol102220e | |
 (2-methylallyl)triphenylstannane | dichloromethane | N Parameter: 5.13 sN Parameter: 0.90 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
 (2-methylallyl)triphenylsilane | dichloromethane | N Parameter: 4.17 sN Parameter: 0.79 | J. Am. Chem. Soc. 2001, 123, 9500-9512 DOI: 10.1021/ja010890y | |
 (2-methylallyl)triphenylgermane | dichloromethane | N Parameter: 4.67 sN Parameter: 0.81 | J. Am. Chem. Soc. 2001, 123, 9500-9512 DOI: 10.1021/ja010890y | |
 (2-methylallyl)trimethylsilane | dichloromethane | N Parameter: 4.41 sN Parameter: 0.96 | J. Am. Chem. Soc. 2001, 123, 9500-9512 DOI: 10.1021/ja010890y | |
 (2-methylallyl)trichlorosilane | dichloromethane | N Parameter: -0.57 sN Parameter: 0.95 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
 (2-methylallyl)tributylstannane | dichloromethane | N Parameter: 7.48 sN Parameter: 0.89 | J. Am. Chem. Soc. 2001, 123, 9500-9512 DOI: 10.1021/ja010890y | |
 (2-methylallyl)dicarbonyl(cyclopentadienyl)iron(II) | dichloromethane | N Parameter: 8.45 sN Parameter: 0.83 | Helv. Chim. Acta 2005, 88, 1754-1768 DOI: 10.1002/hlca.200590137 | |
 (2-methylallyl)benzene | dichloromethane | N Parameter: 0.25 sN Parameter: 1.00 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
 (2,3-dimethylbut-2-enyl)triethylsilane | dichloromethane | N Parameter: 3.15 sN Parameter: 1.15 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
 (1S)-(-)-alpha-pinene | dichloromethane | N Parameter: 0.68 sN Parameter: 1.10 | Acc. Chem. Res. 2003, 36, 66-77 DOI: 10.1021/ar020094c | |
 (1H-inden-1-yl)zinc(II) chloride*LiCl (in DMSO) | DMSO | N Parameter: 18.10 sN Parameter: 0.46 | Angew. Chem. Int. Ed. 2015, 54, 12497-12500 DOI: 10.1002/anie.201501385 | |
 (1H-inden-1-yl)zinc(II) bromide*LiBr (in DMSO) | DMSO | N Parameter: 15.60 sN Parameter: 0.51 | Angew. Chem. Int. Ed. 2015, 54, 12497-12500 DOI: 10.1002/anie.201501385 | |
 (1H-inden-1-yl)trimethylstannane (in CH2Cl2) | dichloromethane | N Parameter: 6.68 sN Parameter: 0.81 | Angew. Chem. Int. Ed. 2015, 54, 12497-12500 DOI: 10.1002/anie.201501385 | |
 (1H-inden-1-yl)trimethylsilane (in CH2Cl2) | dichloromethane | N Parameter: -0.10 sN Parameter: 1.05 | Angew. Chem. Int. Ed. 2015, 54, 12497-12500 DOI: 10.1002/anie.201501385 | |
 potassium trifluoro(furan-2-yl)borate | MeCN | N Parameter: 5.99 sN Parameter: 0.79 | J. Am. Chem. Soc. 2013, 135, 6317-6324 DOI: 10.1021/ja4017655 | |
 lithium (5-methylthiophen-2-yl)(4-(trifluoromethyl)phenyl)pinacolborate | MeCN | N Parameter: 6.24 sN Parameter: 1.00 | Angew. Chem. Int. Ed. 2015, 54, 2780-2783 DOI: 10.1002/anie.201410562 |