
tol(t-Bu)2QM | 0 | E Parameter: -15.83
|     | Angew. Chem. Int. Ed. 2002, 41, 91-95
DOI: 10.1002/1521-3773(20020104)41:1<91::AID-ANIE91>3.0.CO;2-P |

tert-butyl prop-2-enoate (in DMSO) | DMSO | E Parameter: -20.22
|    | J. Am. Chem. Soc. 2017, 139, 13318-13329
DOI: 10.1021/jacs.7b05106 |

p-(methoxy)benzylidenemalononitrile | 0 | E Parameter: -10.80
|    | J. Org. Chem. 2003, 68, 6880-6886
DOI: 10.1021/jo0344182 |

p-(methoxy)benzylidene Meldrum's acid | 0 | E Parameter: -10.28
|    | J. Org. Chem. 2008, 73, 2738-2745
DOI: 10.1021/jo702590s |

p-(dimethylamino)benzylidenemalononitrile | 0 | E Parameter: -13.30
|    | J. Org. Chem. 2003, 68, 6880-6886
DOI: 10.1021/jo0344182 |

p-(dimethylamino)benzylidene Meldrum's acid | 0 | E Parameter: -12.76
|    | J. Org. Chem. 2008, 73, 2738-2745
DOI: 10.1021/jo702590s |

N-methyl maleimide | DMSO | E Parameter: -14.07
|    | Eur. J. Org. Chem. 2014, , 2956-2963
DOI: 10.1002/ejoc.201301779 |

N,N-dimethylprop-2-enamide (in DMSO) | DMSO | E Parameter: -23.54
|    | J. Am. Chem. Soc. 2017, 139, 13318-13329
DOI: 10.1021/jacs.7b05106 |

methyl prop-2-enoate (in DMSO) | DMSO | E Parameter: -18.84
|    | J. Am. Chem. Soc. 2017, 139, 13318-13329
DOI: 10.1021/jacs.7b05106 |

maleic anhydride | DMSO | E Parameter: -11.31
|    | Eur. J. Org. Chem. 2014, , 2956-2963
DOI: 10.1002/ejoc.201301779 |

julBBS | 0 | E Parameter: -13.84
|    | J. Org. Chem. 2007, 72, 9170-9180
DOI: 10.1021/jo071273g |

jul-indan-1,3-dione | 0 | E Parameter: -14.68
|    | Org. Biomol. Chem. 2007, 5, 3020-3026
DOI: 10.1039/b708025e |

jul(t-Bu)2QM | 0 | E Parameter: -17.90
|     | Angew. Chem. Int. Ed. 2002, 41, 91-95
DOI: 10.1002/1521-3773(20020104)41:1<91::AID-ANIE91>3.0.CO;2-P |

jul(S)BBS | 0 | E Parameter: -11.89
|    | J. Org. Chem. 2007, 72, 9170-9180
DOI: 10.1021/jo071273g |

jul Meldrum's acid | 0 | E Parameter: -13.97
|    | J. Org. Chem. 2008, 73, 2738-2745
DOI: 10.1021/jo702590s |

hex-3-yn-2-one | 0 | E Parameter: -17.90
|  | Angew. Chem. Int. Ed. 2019, 58, 17704-17708
DOI: 10.1002/anie.201909803 |

furan-2(5H)-one | DMSO | E Parameter: -20.70
|    | Chem. Sci. 2021, 12, 4850-4865
DOI: 10.1039/D0SC06628A |

fumaronitrile | DMSO | E Parameter: -15.71
|    | Eur. J. Org. Chem. 2014, , 2956-2963
DOI: 10.1002/ejoc.201301779 |

ethyl prop-2-enoate (in DMSO) | DMSO | E Parameter: -19.07
|    | J. Am. Chem. Soc. 2017, 139, 13318-13329
DOI: 10.1021/jacs.7b05106 |

ethyl cinnamate (in DMSO) | DMSO | E Parameter: -24.52
|   | J. Am. Chem. Soc. 2017, 139, 13318-13329
DOI: 10.1021/jacs.7b05106 |

ethyl 2-methylprop-2-enoate (in DMSO) | DMSO | E Parameter: -22.77
|   | J. Am. Chem. Soc. 2017, 139, 13318-13329
DOI: 10.1021/jacs.7b05106 |
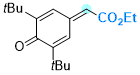
ethyl 2-(3,5-di-tert-butyl-4-oxocyclohexa-2,5-dien-1-ylidene)acetate | 0 | E Parameter: -12.01
|    | Chem. Eur. J. 2025, 31, e202501224
DOI: 10.1002/chem.202501224 |

ethyl (E)-but-2-enoate (in DMSO) | DMSO | E Parameter: -23.59
|   | J. Am. Chem. Soc. 2017, 139, 13318-13329
DOI: 10.1021/jacs.7b05106 |

ethenylsulfonylbenzene (in DMSO) | DMSO | E Parameter: -18.36
|    | J. Am. Chem. Soc. 2017, 139, 13318-13329
DOI: 10.1021/jacs.7b05106 |

ethenesulfonyl fluoride (ESF) | DMSO | E Parameter: -12.09
|    | Angew. Chem. Int. Ed. 2016, 55, 12664-12667
DOI: 10.1002/anie.201601875 |

E)-2,6-di-tert-butyl-4-(3-(4-chlorophenyl)allylidene)cyclohexa-2,5-dien-1-one | DMSO | E Parameter: -16.84
|    | Org. Lett. 2020, 22, 2182-2186
DOI: 10.1021/acs.orglett.0c00338 |

dmaBBS | 0 | E Parameter: -12.76
|    | J. Org. Chem. 2007, 72, 9170-9180
DOI: 10.1021/jo071273g |

dma(t-Bu)2QM | | E Parameter: -17.29
|     | Angew. Chem. Int. Ed. 2002, 41, 91-95
DOI: 10.1002/1521-3773(20020104)41:1<91::AID-ANIE91>3.0.CO;2-P |

dma(S)BBS | 0 | E Parameter: -10.73
|    | J. Org. Chem. 2007, 72, 9170-9180
DOI: 10.1021/jo071273g |

dma(Ph)2QM | 0 | E Parameter: -13.39
|     | Angew. Chem. Int. Ed. 2002, 41, 91-95
DOI: 10.1002/1521-3773(20020104)41:1<91::AID-ANIE91>3.0.CO;2-P |

dihydro-2H-pyran-2-one | DMSO | E Parameter: -21.80
|   | Chem. Sci. 2021, 12, 4850-4865
DOI: 10.1039/D0SC06628A |

diethyl maleate | DMSO | E Parameter: -19.49
|    | Eur. J. Org. Chem. 2014, , 2956-2963
DOI: 10.1002/ejoc.201301779 |

diethyl fumarate | DMSO | E Parameter: -17.79
|    | Eur. J. Org. Chem. 2014, , 2956-2963
DOI: 10.1002/ejoc.201301779 |

diethyl benzylidene malonate | 0 | E Parameter: -20.55
|    | Chem. Eur. J. 2008, 14, 9675-9682
DOI: 10.1002/chem.200801277 |

diethyl 4-nitrobenzylidene malonate | 0 | E Parameter: -17.67
|    | Chem. Eur. J. 2008, 14, 9675-9682
DOI: 10.1002/chem.200801277 |

diethyl 4-methyl-benzylidene malonate | 0 | E Parameter: -21.11
|    | Chem. Eur. J. 2008, 14, 9675-9682
DOI: 10.1002/chem.200801277 |

diethyl 4-methoxy-benzylidene malonate | 0 | E Parameter: -21.47
|    | Chem. Eur. J. 2008, 14, 9675-9682
DOI: 10.1002/chem.200801277 |

diethyl 4-cyano-benzylidene malonate | 0 | E Parameter: -18.06
|    | Chem. Eur. J. 2008, 14, 9675-9682
DOI: 10.1002/chem.200801277 |

diethyl 4-(dimethylamino)benzylidene malonate | 0 | E Parameter: -23.10
|    | Chem. Eur. J. 2008, 14, 9675-9682
DOI: 10.1002/chem.200801277 |

diethyl 3-chloro-benzylidene malonate | 0 | E Parameter: -18.98
|    | Chem. Eur. J. 2008, 14, 9675-9682
DOI: 10.1002/chem.200801277 |

diethyl 2-((julolidine-9-yl)methylene)malonate | 0 | E Parameter: -23.80
|  | Chem. Eur. J. 2008, 14, 9675-9682
DOI: 10.1002/chem.200801277 |

diethyl 2-((1-methyl-1,2,3,4-tetrahydroquinolin-6-yl)methylene)malonate | 0 | E Parameter: -23.40
|  | Chem. Eur. J. 2008, 14, 9675-9682
DOI: 10.1002/chem.200801277 |

cyclopentenone | DMSO | E Parameter: -20.60
|    | Chem. Sci. 2021, 12, 4850-4865
DOI: 10.1039/D0SC06628A |

cyclohexenone | DMSO | E Parameter: -22.10
|    | Chem. Sci. 2021, 12, 4850-4865
DOI: 10.1039/D0SC06628A |

cycloheptenone | DMSO | E Parameter: -22.00
|    | Chem. Sci. 2021, 12, 4850-4865
DOI: 10.1039/D0SC06628A |

cinnamonitrile (in DMSO) | DMSO | E Parameter: -24.60
|  | J. Am. Chem. Soc. 2017, 139, 13318-13329
DOI: 10.1021/jacs.7b05106 |

butynone | 0 | E Parameter: -16.60
|  | Angew. Chem. Int. Ed. 2019, 58, 17704-17708
DOI: 10.1002/anie.201909803 |

but-3-en-2-one (in DMSO) | DMSO | E Parameter: -16.76
|    | J. Am. Chem. Soc. 2017, 139, 13318-13329
DOI: 10.1021/jacs.7b05106 |

bh6j | | E Parameter: -17.90
|   | Chem. Eur. J. 2010, 16, 1365-1371
DOI: 10.1002/chem.200902487 |

bh6a | | E Parameter: -18.60
|   | Chem. Eur. J. 2010, 16, 1365-1371
DOI: 10.1002/chem.200902487 |

bh 6m | | E Parameter: -17.30
|  | Chem. Eur. J. 2010, 16, 1365-1371
DOI: 10.1002/chem.200902487 |

bh 6l | | E Parameter: -18.90
|  | Chem. Eur. J. 2010, 16, 1365-1371
DOI: 10.1002/chem.200902487 |

bh 6k | | E Parameter: -18.20
|  | Chem. Eur. J. 2010, 16, 1365-1371
DOI: 10.1002/chem.200902487 |

bh 6i | | E Parameter: -19.10
|   | Chem. Eur. J. 2010, 16, 1365-1371
DOI: 10.1002/chem.200902487 |

bh 6f | | E Parameter: -18.90
|   | Chem. Eur. J. 2010, 16, 1365-1371
DOI: 10.1002/chem.200902487 |

benzylidenemalononitrile | 0 | E Parameter: -9.42
|    | J. Org. Chem. 2003, 68, 6880-6886
DOI: 10.1021/jo0344182 |

benzylidene-Meldrum's acid | 0 | E Parameter: -9.15
|    | J. Org. Chem. 2008, 73, 2738-2745
DOI: 10.1021/jo702590s |

aniBBS | 0 | E Parameter: -10.37
|    | J. Org. Chem. 2007, 72, 9170-9180
DOI: 10.1021/jo071273g |

ani(t-Bu)2QM | | E Parameter: -16.11
|     | Angew. Chem. Int. Ed. 2002, 41, 91-95
DOI: 10.1002/1521-3773(20020104)41:1<91::AID-ANIE91>3.0.CO;2-P |

ani(Ph)2QM | 0 | E Parameter: -12.18
|     | Angew. Chem. Int. Ed. 2002, 41, 91-95
DOI: 10.1002/1521-3773(20020104)41:1<91::AID-ANIE91>3.0.CO;2-P |

ani(Br)2QM | 0 | E Parameter: -8.63
|     | Angew. Chem. Int. Ed. 2002, 41, 91-95
DOI: 10.1002/1521-3773(20020104)41:1<91::AID-ANIE91>3.0.CO;2-P |

acrylonitrile (in DMSO) | DMSO | E Parameter: -19.05
|    | J. Am. Chem. Soc. 2017, 139, 13318-13329
DOI: 10.1021/jacs.7b05106 |

<i>trans-beta</i>-nitrostyrene | | E Parameter: -13.85
|    | J. Org. Chem. 2011, 76, 9370-9378
DOI: 10.1021/jo201678u |

4-phenylbut-3-yn-2-one | 0 | E Parameter: -16.90
|  | Angew. Chem. Int. Ed. 2019, 58, 17704-17708
DOI: 10.1002/anie.201909803 |

4-nitro-<i>trans-beta</i>-nitrostyrene | | E Parameter: -12.37
|    | J. Org. Chem. 2011, 76, 9370-9378
DOI: 10.1021/jo201678u |

4-methyl-<i>trans-beta</i>-nitrostyrene | | E Parameter: -14.23
|    | J. Org. Chem. 2011, 76, 9370-9378
DOI: 10.1021/jo201678u |

4-methoxy-<i>trans-beta</i>-nitrostyrene | | E Parameter: -14.70
|    | J. Org. Chem. 2011, 76, 9370-9378
DOI: 10.1021/jo201678u |

4-cyano-<i>trans-beta</i>-nitrostyrene | | E Parameter: -12.61
|    | J. Org. Chem. 2011, 76, 9370-9378
DOI: 10.1021/jo201678u |

4-bromo-<i>trans-beta</i>-nitrostyrene | | E Parameter: -13.37
|    | J. Org. Chem. 2011, 76, 9370-9378
DOI: 10.1021/jo201678u |
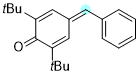
4-benzylidene-2,6-di-tert-butylcyclohexa-2,5-dien-1-one | 0 | E Parameter: -15.58
|    | Chem. Eur. J. 2025, 31, e202501224
DOI: 10.1002/chem.202501224 |

4-(2,2-bis(phenylsulfonyl)vinyl)-N,N-dimethylaniline | DMSO | E Parameter: -16.53
|    | Chem. Asian J. 2012, 7, 1401-1407
DOI: 10.1002/asia.201101046 |

3-methylenetetrahydropyran-2-one | DMSO | E Parameter: -19.50
|    | Chem. Sci. 2021, 12, 4850-4865
DOI: 10.1039/D0SC06628A |

3-methylenedihydrofuranone | DMSO | E Parameter: -19.40
|    | Chem. Sci. 2021, 12, 4850-4865
DOI: 10.1039/D0SC06628A |

3-methylcyclopentenone | DMSO | E Parameter: -28.90
|  | Chem. Sci. 2021, 12, 4850-4865
DOI: 10.1039/D0SC06628A |

3-methylcyclohexenone | DMSO | E Parameter: -29.60
|  | Chem. Sci. 2021, 12, 4850-4865
DOI: 10.1039/D0SC06628A |

2-phenylethene-1-sulfonyl fluoride | DMSO | E Parameter: -16.63
|    | Angew. Chem. Int. Ed. 2016, 55, 12664-12667
DOI: 10.1002/anie.201601875 |

2-methylcyclopentenone | DMSO | E Parameter: -22.10
|    | Chem. Sci. 2021, 12, 4850-4865
DOI: 10.1039/D0SC06628A |

2-methylcyclohexenone | DMSO | E Parameter: -27.50
|  | Chem. Sci. 2021, 12, 4850-4865
DOI: 10.1039/D0SC06628A |

2-cinnamoyl-3-methyl-1-(perfluorophenyl)-1H-imidazol-3-ium | DMSO | E Parameter: -10.09
|    | Top. Catal. 2018, 61, 585-590
DOI: 10.1007/s11244-018-0914-5 |

2-cinnamoyl-1-mesityl-3-methyl-1H-imidazol-3-ium | DMSO | E Parameter: -11.48
|    | Top. Catal. 2018, 61, 585-590
DOI: 10.1007/s11244-018-0914-5 |

2-cinnamoyl-1-(4-methoxyphenyl)-3-methyl-1H-imidazol-3-ium | DMSO | E Parameter: -11.79
|    | Top. Catal. 2018, 61, 585-590
DOI: 10.1007/s11244-018-0914-5 |

2-cinnamoyl-1-(2,6-dimethoxyphenyl)-3-methyl-1H-imidazol-3-ium | DMSO | E Parameter: -12.02
|    | Top. Catal. 2018, 61, 585-590
DOI: 10.1007/s11244-018-0914-5 |

2-cinnamoyl-1,3-dimethyl-1H-imidazol-3-ium | DMSO | E Parameter: -11.52
|    | Angew. Chem. Int. Ed. 2012, 51, 5234-5238
DOI: 10.1002/anie.201109042 |

2-benzylidenebenzo[d][1,3]dithiole 1,1,3,3-tetraoxide | DMSO | E Parameter: -11.78
|    | Chem. Asian J. 2012, 7, 1401-1407
DOI: 10.1002/asia.201101046 |

2-benzylidene-indan-1,3-dione | 0 | E Parameter: -10.11
|    | Org. Biomol. Chem. 2007, 5, 3020-3026
DOI: 10.1039/b708025e |

2-(p-methoxybenzylidene)-indan-1,3-dione | 0 | E Parameter: -11.32
|    | Org. Biomol. Chem. 2007, 5, 3020-3026
DOI: 10.1039/b708025e |

2-(p-(dimethylamino)benzylidene)-indan-1,3-dione | 0 | E Parameter: -13.56
|    | Org. Biomol. Chem. 2007, 5, 3020-3026
DOI: 10.1039/b708025e |

2-(4-methoxybenzylidene)benzo[d][1,3]dithiole 1,1,3,3-tetraoxide | DMSO | E Parameter: -13.02
|    | Chem. Asian J. 2012, 7, 1401-1407
DOI: 10.1002/asia.201101046 |

2-(4-methoxybenzylidene)-1,3-dithiane 1,1,3,3-tetraoxide | DMSO | E Parameter: -14.55
|    | Chem. Asian J. 2012, 7, 1401-1407
DOI: 10.1002/asia.201101046 |
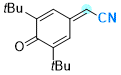
2-(3,5-di-tert-butyl-4-oxocyclohexa-2,5-dien-1-ylidene)acetonitrile | 0 | E Parameter: -11.88
|    | Chem. Eur. J. 2025, 31, e202501224
DOI: 10.1002/chem.202501224 |

2,6-diphenyl-4-benzylidenecyclohexa-2,5-dienone | 0 | E Parameter: -11.87
|    | Eur. J. Org. Chem. 2009, , 3203-3211
DOI: 10.1002/ejoc.200900299 |

2,6-dimethyl-4-(4-(dimethylamino)benzylidene)cyclohexa-2,5-dienone | 0 | E Parameter: -16.36
|    | Eur. J. Org. Chem. 2009, , 3203-3211
DOI: 10.1002/ejoc.200900299 |

2,6-dimethoxy-4-(4-methoxybenzylidene)cyclohexa-2,5-dienone | 0 | E Parameter: -16.38
|    | Eur. J. Org. Chem. 2009, , 3203-3211
DOI: 10.1002/ejoc.200900299 |

2,6-dimethoxy-4-(4-(dimethylamino)benzylidene)cyclohexa-2,5-dienone | 0 | E Parameter: -17.18
|    | Eur. J. Org. Chem. 2009, , 3203-3211
DOI: 10.1002/ejoc.200900299 |

2,6-di-tert.butyl-4-(4-nitrobenzylidene)cyclohexa-2,5-dienone | 0 | E Parameter: -14.36
|    | Eur. J. Org. Chem. 2009, , 3203-3211
DOI: 10.1002/ejoc.200900299 |

2,6-di-tert.butyl-4-(3-fluorobenzylidene)cyclohexa-2,5-dienone | 0 | E Parameter: -15.03
|    | Eur. J. Org. Chem. 2009, , 3203-3211
DOI: 10.1002/ejoc.200900299 |

2,6-di-tert.butyl-4-(3,5-difluorobenzylidene)cyclohexa-2,5-dienone | 0 | E Parameter: -14.50
|    | Eur. J. Org. Chem. 2009, , 3203-3211
DOI: 10.1002/ejoc.200900299 |
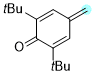
2,6-di-tert-butyl-4-methylenecyclohexa-2,5-dien-1-one | 0 | E Parameter: -11.17
|    | Chem. Eur. J. 2025, 31, e202501224
DOI: 10.1002/chem.202501224 |
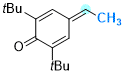
2,6-di-tert-butyl-4-ethylidenecyclohexa-2,5-dien-1-one | 0 | E Parameter: -13.68
|    | Chem. Eur. J. 2025, 31, e202501224
DOI: 10.1002/chem.202501224 |
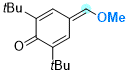
2,6-di-tert-butyl-4-(methoxymethylene)cyclohexa-2,5-dien-1-one | 0 | E Parameter: -15.10
|    | Chem. Eur. J. 2025, 31, e202501224
DOI: 10.1002/chem.202501224 |

2,6-di-tert-butyl-4-(2,2,2-trifluoroethylidene)cyclohexa-2,5-dien-1-one | DMSO | E Parameter: -11.68
|    | Eur. J. Org. Chem. 2020, , 3812-3817
DOI: 10.1002/ejoc.202000295 |

1-phenylprop-2-en-1-one (in DMSO) | DMSO | E Parameter: -15.25
|    | J. Am. Chem. Soc. 2017, 139, 13318-13329
DOI: 10.1021/jacs.7b05106 |

1-(tert-butyl)-2-cinnamoyl-3-methyl-1H-imidazol-3-ium | DMSO | E Parameter: -11.80
|    | Top. Catal. 2018, 61, 585-590
DOI: 10.1007/s11244-018-0914-5 |

1,2-diaza-1,3-diene 1f | MeOH | E Parameter: -15.30
|  | Chem. Eur. J. 2010, 16, 12008-12016
DOI: 10.1002/chem.201000828 |

1,2-diaza-1,3-diene 1e | MeOH | E Parameter: -14.90
|  | Chem. Eur. J. 2010, 16, 12008-12016
DOI: 10.1002/chem.201000828 |

1,2-diaza-1,3-diene 1d | DMSO | E Parameter: -15.38
|    | Chem. Eur. J. 2010, 16, 12008-12016
DOI: 10.1002/chem.201000828 |

1,2-diaza-1,3-diene 1c | DMSO | E Parameter: -14.91
|    | Chem. Eur. J. 2010, 16, 12008-12016
DOI: 10.1002/chem.201000828 |

1,2-diaza-1,3-diene 1b | DMSO | E Parameter: -13.90
|    | Chem. Eur. J. 2010, 16, 12008-12016
DOI: 10.1002/chem.201000828 |

1,2-diaza-1,3-diene 1a | DMSO | E Parameter: -13.28
|    | Chem. Eur. J. 2010, 16, 12008-12016
DOI: 10.1002/chem.201000828 |

(ethene-1,1-diyldisulfonyl)dibenzene | DMSO | E Parameter: -7.50
|    | Chem. Asian J. 2012, 7, 1401-1407
DOI: 10.1002/asia.201101046 |
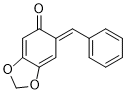
(E)-6-benzylidenebenzo[d][1,3]dioxol-5(6H)-one | 0 | E Parameter: -14.47
|    | Chem. Eur. J. 2025, 31, e202403785
DOI: 10.1002/chem.202403785 |
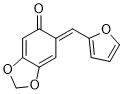
(E)-6-(furan-2-ylmethylene)benzo[d][1,3]dioxol-5(6H)-one | 0 | E Parameter: -15.73
|    | Org. Biomol. Chem. 2025, 23, 827-834
DOI: 10.1039/D4OB01855A |
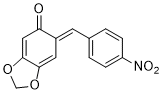
(E)-6-(4-nitrobenzylidene)benzo[d][1,3]dioxol-5(6H)-one | 0 | E Parameter: -13.15
|    | Chem. Eur. J. 2025, 31, e202403785
DOI: 10.1002/chem.202403785 |
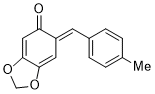
(E)-6-(4-methylbenzylidene)benzo[d][1,3]dioxol-5(6H)-one | 0 | E Parameter: -14.85
|    | Chem. Eur. J. 2025, 31, e202403785
DOI: 10.1002/chem.202403785 |
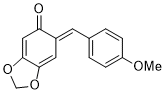
(E)-6-(4-methoxybenzylidene)benzo[d][1,3]dioxol-5(6H)-one | 0 | E Parameter: -15.20
|    | Chem. Eur. J. 2025, 31, e202403785
DOI: 10.1002/chem.202403785 |
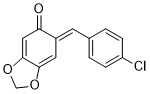
(E)-6-(4-chlorobenzylidene)benzo[d][1,3]dioxol-5(6H)-one | 0 | E Parameter: -13.99
|    | Chem. Eur. J. 2025, 31, e202403785
DOI: 10.1002/chem.202403785 |
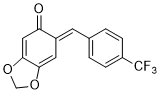
(E)-6-(4-(trifluoromethyl)benzylidene)benzo[d][1,3]dioxol-5(6H)-one | 0 | E Parameter: -13.54
|    | Chem. Eur. J. 2025, 31, e202403785
DOI: 10.1002/chem.202403785 |
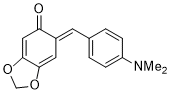
(E)-6-(4-(dimethylamino)benzylidene)benzo[d][1,3]dioxol-5(6H)-one | 0 | E Parameter: -16.07
|    | Chem. Eur. J. 2025, 31, e202403785
DOI: 10.1002/chem.202403785 |
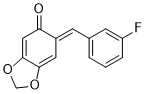
(E)-6-(3-fluorobenzylidene)benzo[d][1,3]dioxol-5(6H)-one | 0 | E Parameter: -14.00
|    | Chem. Eur. J. 2025, 31, e202403785
DOI: 10.1002/chem.202403785 |
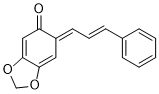
(E)-6-((E)-3-phenylallylidene)benzo[d][1,3]dioxol-5(6H)-one | 0 | E Parameter: -16.00
|    | Org. Biomol. Chem. 2025, 23, 827-834
DOI: 10.1039/D4OB01855A |
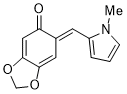
(E)-6-((1-methyl-1H-pyrrol-2-yl)methylene)benzo[d][1,3]dioxol-5(6H)-one | 0 | E Parameter: -17.03
|    | Org. Biomol. Chem. 2025, 23, 827-834
DOI: 10.1039/D4OB01855A |
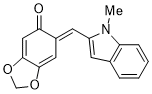
(E)-6-((1-methyl-1H-indol-2-yl)methylene)benzo[d][1,3]dioxol-5(6H)-one | 0 | E Parameter: -15.55
|    | Org. Biomol. Chem. 2025, 23, 827-834
DOI: 10.1039/D4OB01855A |

(E)-4-phenylbut-3-en-2-one (in DMSO) | DMSO | E Parameter: -23.01
|    | J. Am. Chem. Soc. 2017, 139, 13318-13329
DOI: 10.1021/jacs.7b05106 |

(E)-4-methyl-1-(4-nitrophenyl)pent-1-en-3-one (in DMSO) | DMSO | E Parameter: -19.17
|  | J. Am. Chem. Soc. 2011, 133, 8240-8251
DOI: 10.1021/ja200820m |

(E)-4-(4-nitrophenyl)but-3-en-2-one (in DSMO) | DMSO | E Parameter: -19.36
|  | J. Am. Chem. Soc. 2011, 133, 8240-8251
DOI: 10.1021/ja200820m |

(E)-4,4-dimethyl-1-(4-nitrophenyl)pent-1-en-3-one | DMSO | E Parameter: -19.15
|  | J. Am. Chem. Soc. 2011, 133, 8240-8251
DOI: 10.1021/ja200820m |

(E)-3-(4-nitro-phenyl)-1-phenyl-prop-2-en-1-one (in DMSO) | DMSO | E Parameter: -17.33
|    | J. Am. Chem. Soc. 2011, 133, 8240-8251
DOI: 10.1021/ja200820m |

(E)-3-(4-cyano-phenyl)-1-phenyl-prop-2-en-1-one (in DMSO) | DMSO | E Parameter: -17.64
|    | J. Am. Chem. Soc. 2011, 133, 8240-8251
DOI: 10.1021/ja200820m |
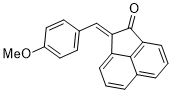
(E)-2-(4-methoxybenzylidene)acenaphthylen-1(2H)-one | 0 | E Parameter: -18.72
|    | J. Phys. Org. Chem. 2025, , accepted
DOI: 10.1002/poc.70061 |

(E)-2,6-di-tert-butyl-4-(3-phenylallylidene)cyclohexa-2,5-dien-1-one | DMSO | E Parameter: -17.00
|    | Org. Lett. 2020, 22, 2182-2186
DOI: 10.1021/acs.orglett.0c00338 |

(E)-2,6-di-tert-butyl-4-(3-(4-nitrophenyl)allylidene)cyclohexa-2,5-dien-1-one | DMSO | E Parameter: -16.25
|    | Org. Lett. 2020, 22, 2182-2186
DOI: 10.1021/acs.orglett.0c00338 |

(E)-2,6-di-tert-butyl-4-(3-(4-methoxyphenyl)allylidene)cyclohexa-2,5-dien-1-one | DMSO | E Parameter: -17.42
|    | Org. Lett. 2020, 22, 2182-2186
DOI: 10.1021/acs.orglett.0c00338 |
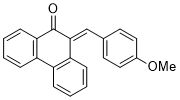
(E)-10-(4-methoxybenzylidene)phenanthren-9(10H)-one | 0 | E Parameter: -15.93
|    | J. Phys. Org. Chem. 2025, , accepted
DOI: 10.1002/poc.70061 |

(E)-1-methyl-4-(styrylsulfonyl)benzene (in DMSO) | DMSO | E Parameter: -24.69
|   | J. Am. Chem. Soc. 2017, 139, 13318-13329
DOI: 10.1021/jacs.7b05106 |

(E)-1,3-diphenylprop-2-en-1-one (in DMSO) | DMSO | E Parameter: -19.39
|    | J. Am. Chem. Soc. 2017, 139, 13318-13329
DOI: 10.1021/jacs.7b05106 |

(2-phenylethene-1,1-diyldisulfonyl)dibenzene | DMSO | E Parameter: -12.93
|    | Chem. Asian J. 2012, 7, 1401-1407
DOI: 10.1002/asia.201101046 |

(2-(4-methoxyphenyl)ethene-1,1-diyldisulfonyl)dibenzene | DMSO | E Parameter: -13.88
|    | Chem. Asian J. 2012, 7, 1401-1407
DOI: 10.1002/asia.201101046 |