Other Michael acceptors
For reactivities of structurally related compounds go to: Electrophiles » C-Electrophiles » Acceptor-Substituted Ethylenes
Found 58 Molecules.
| Molecule | Solvent | Reactivity Parameters | Classification | Reference (sort by title or year) |
|---|---|---|---|---|
 3-methylcyclohexenone | DMSO | E Parameter: -29.60 | Chem. Sci. 2021, 12, 4850-4865 DOI: 10.1039/D0SC06628A | |
 3-methylcyclopentenone | DMSO | E Parameter: -28.90 | Chem. Sci. 2021, 12, 4850-4865 DOI: 10.1039/D0SC06628A | |
 2-methylcyclohexenone | DMSO | E Parameter: -27.50 | Chem. Sci. 2021, 12, 4850-4865 DOI: 10.1039/D0SC06628A | |
 (E)-1-methyl-4-(styrylsulfonyl)benzene (in DMSO) | DMSO | E Parameter: -24.69 | J. Am. Chem. Soc. 2017, 139, 13318-13329 DOI: 10.1021/jacs.7b05106 | |
 cinnamonitrile (in DMSO) | DMSO | E Parameter: -24.60 | J. Am. Chem. Soc. 2017, 139, 13318-13329 DOI: 10.1021/jacs.7b05106 | |
 ethyl cinnamate (in DMSO) | DMSO | E Parameter: -24.52 | J. Am. Chem. Soc. 2017, 139, 13318-13329 DOI: 10.1021/jacs.7b05106 | |
 ethyl (E)-but-2-enoate (in DMSO) | DMSO | E Parameter: -23.59 | J. Am. Chem. Soc. 2017, 139, 13318-13329 DOI: 10.1021/jacs.7b05106 | |
 N,N-dimethylprop-2-enamide (in DMSO) | DMSO | E Parameter: -23.54 | J. Am. Chem. Soc. 2017, 139, 13318-13329 DOI: 10.1021/jacs.7b05106 | |
 (E)-4-phenylbut-3-en-2-one (in DMSO) | DMSO | E Parameter: -23.01 | J. Am. Chem. Soc. 2017, 139, 13318-13329 DOI: 10.1021/jacs.7b05106 | |
 ethyl 2-methylprop-2-enoate (in DMSO) | DMSO | E Parameter: -22.77 | J. Am. Chem. Soc. 2017, 139, 13318-13329 DOI: 10.1021/jacs.7b05106 | |
 2-methylcyclopentenone | DMSO | E Parameter: -22.10 | Chem. Sci. 2021, 12, 4850-4865 DOI: 10.1039/D0SC06628A | |
 cyclohexenone | DMSO | E Parameter: -22.10 | Chem. Sci. 2021, 12, 4850-4865 DOI: 10.1039/D0SC06628A | |
 cycloheptenone | DMSO | E Parameter: -22.00 | Chem. Sci. 2021, 12, 4850-4865 DOI: 10.1039/D0SC06628A | |
 dihydro-2H-pyran-2-one | DMSO | E Parameter: -21.80 | Chem. Sci. 2021, 12, 4850-4865 DOI: 10.1039/D0SC06628A | |
 furan-2(5H)-one | DMSO | E Parameter: -20.70 | Chem. Sci. 2021, 12, 4850-4865 DOI: 10.1039/D0SC06628A | |
 cyclopentenone | DMSO | E Parameter: -20.60 | Chem. Sci. 2021, 12, 4850-4865 DOI: 10.1039/D0SC06628A | |
 tert-butyl prop-2-enoate (in DMSO) | DMSO | E Parameter: -20.22 | J. Am. Chem. Soc. 2017, 139, 13318-13329 DOI: 10.1021/jacs.7b05106 | |
 3-methylenetetrahydropyran-2-one | DMSO | E Parameter: -19.50 | Chem. Sci. 2021, 12, 4850-4865 DOI: 10.1039/D0SC06628A | |
 diethyl maleate | DMSO | E Parameter: -19.49 | Eur. J. Org. Chem. 2014, , 2956-2963 DOI: 10.1002/ejoc.201301779 | |
 3-methylenedihydrofuranone | DMSO | E Parameter: -19.40 | Chem. Sci. 2021, 12, 4850-4865 DOI: 10.1039/D0SC06628A | |
 (E)-1,3-diphenylprop-2-en-1-one (in DMSO) | DMSO | E Parameter: -19.39 | J. Am. Chem. Soc. 2017, 139, 13318-13329 DOI: 10.1021/jacs.7b05106 | |
 (E)-4-(4-nitrophenyl)but-3-en-2-one (in DSMO) | DMSO | E Parameter: -19.36 | J. Am. Chem. Soc. 2011, 133, 8240-8251 DOI: 10.1021/ja200820m | |
 (E)-4-methyl-1-(4-nitrophenyl)pent-1-en-3-one (in DMSO) | DMSO | E Parameter: -19.17 | J. Am. Chem. Soc. 2011, 133, 8240-8251 DOI: 10.1021/ja200820m | |
 (E)-4,4-dimethyl-1-(4-nitrophenyl)pent-1-en-3-one | DMSO | E Parameter: -19.15 | J. Am. Chem. Soc. 2011, 133, 8240-8251 DOI: 10.1021/ja200820m | |
 ethyl prop-2-enoate (in DMSO) | DMSO | E Parameter: -19.07 | J. Am. Chem. Soc. 2017, 139, 13318-13329 DOI: 10.1021/jacs.7b05106 | |
 acrylonitrile (in DMSO) | DMSO | E Parameter: -19.05 | J. Am. Chem. Soc. 2017, 139, 13318-13329 DOI: 10.1021/jacs.7b05106 | |
 methyl prop-2-enoate (in DMSO) | DMSO | E Parameter: -18.84 | J. Am. Chem. Soc. 2017, 139, 13318-13329 DOI: 10.1021/jacs.7b05106 | |
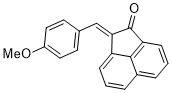 (E)-2-(4-methoxybenzylidene)acenaphthylen-1(2H)-one | 0 | E Parameter: -18.72 | J. Phys. Org. Chem. 2025, , accepted DOI: 10.1002/poc.70061 | |
 ethenylsulfonylbenzene (in DMSO) | DMSO | E Parameter: -18.36 | J. Am. Chem. Soc. 2017, 139, 13318-13329 DOI: 10.1021/jacs.7b05106 | |
 hex-3-yn-2-one | 0 | E Parameter: -17.90 | Angew. Chem. Int. Ed. 2019, 58, 17704-17708 DOI: 10.1002/anie.201909803 | |
 diethyl fumarate | DMSO | E Parameter: -17.79 | Eur. J. Org. Chem. 2014, , 2956-2963 DOI: 10.1002/ejoc.201301779 | |
 (E)-3-(4-cyano-phenyl)-1-phenyl-prop-2-en-1-one (in DMSO) | DMSO | E Parameter: -17.64 | J. Am. Chem. Soc. 2011, 133, 8240-8251 DOI: 10.1021/ja200820m | |
 (E)-3-(4-nitro-phenyl)-1-phenyl-prop-2-en-1-one (in DMSO) | DMSO | E Parameter: -17.33 | J. Am. Chem. Soc. 2011, 133, 8240-8251 DOI: 10.1021/ja200820m | |
 4-phenylbut-3-yn-2-one | 0 | E Parameter: -16.90 | Angew. Chem. Int. Ed. 2019, 58, 17704-17708 DOI: 10.1002/anie.201909803 | |
 but-3-en-2-one (in DMSO) | DMSO | E Parameter: -16.76 | J. Am. Chem. Soc. 2017, 139, 13318-13329 DOI: 10.1021/jacs.7b05106 | |
 2-phenylethene-1-sulfonyl fluoride | DMSO | E Parameter: -16.63 | Angew. Chem. Int. Ed. 2016, 55, 12664-12667 DOI: 10.1002/anie.201601875 | |
 butynone | 0 | E Parameter: -16.60 | Angew. Chem. Int. Ed. 2019, 58, 17704-17708 DOI: 10.1002/anie.201909803 | |
 fumaronitrile | DMSO | E Parameter: -15.71 | Eur. J. Org. Chem. 2014, , 2956-2963 DOI: 10.1002/ejoc.201301779 | |
 1-phenylprop-2-en-1-one (in DMSO) | DMSO | E Parameter: -15.25 | J. Am. Chem. Soc. 2017, 139, 13318-13329 DOI: 10.1021/jacs.7b05106 | |
 jul-indan-1,3-dione | 0 | E Parameter: -14.68 | Org. Biomol. Chem. 2007, 5, 3020-3026 DOI: 10.1039/b708025e | |
 N-methyl maleimide | DMSO | E Parameter: -14.07 | Eur. J. Org. Chem. 2014, , 2956-2963 DOI: 10.1002/ejoc.201301779 | |
 jul Meldrum's acid | 0 | E Parameter: -13.97 | J. Org. Chem. 2008, 73, 2738-2745 DOI: 10.1021/jo702590s | |
 julBBS | 0 | E Parameter: -13.84 | J. Org. Chem. 2007, 72, 9170-9180 DOI: 10.1021/jo071273g | |
 2-(p-(dimethylamino)benzylidene)-indan-1,3-dione | 0 | E Parameter: -13.56 | Org. Biomol. Chem. 2007, 5, 3020-3026 DOI: 10.1039/b708025e | |
 p-(dimethylamino)benzylidenemalononitrile | 0 | E Parameter: -13.30 | J. Org. Chem. 2003, 68, 6880-6886 DOI: 10.1021/jo0344182 | |
 dmaBBS | 0 | E Parameter: -12.76 | J. Org. Chem. 2007, 72, 9170-9180 DOI: 10.1021/jo071273g | |
 p-(dimethylamino)benzylidene Meldrum's acid | 0 | E Parameter: -12.76 | J. Org. Chem. 2008, 73, 2738-2745 DOI: 10.1021/jo702590s | |
 ethenesulfonyl fluoride (ESF) | DMSO | E Parameter: -12.09 | Angew. Chem. Int. Ed. 2016, 55, 12664-12667 DOI: 10.1002/anie.201601875 | |
 jul(S)BBS | 0 | E Parameter: -11.89 | J. Org. Chem. 2007, 72, 9170-9180 DOI: 10.1021/jo071273g | |
 2-(p-methoxybenzylidene)-indan-1,3-dione | 0 | E Parameter: -11.32 | Org. Biomol. Chem. 2007, 5, 3020-3026 DOI: 10.1039/b708025e | |
 maleic anhydride | DMSO | E Parameter: -11.31 | Eur. J. Org. Chem. 2014, , 2956-2963 DOI: 10.1002/ejoc.201301779 | |
 p-(methoxy)benzylidenemalononitrile | 0 | E Parameter: -10.80 | J. Org. Chem. 2003, 68, 6880-6886 DOI: 10.1021/jo0344182 | |
 dma(S)BBS | 0 | E Parameter: -10.73 | J. Org. Chem. 2007, 72, 9170-9180 DOI: 10.1021/jo071273g | |
 aniBBS | 0 | E Parameter: -10.37 | J. Org. Chem. 2007, 72, 9170-9180 DOI: 10.1021/jo071273g | |
 p-(methoxy)benzylidene Meldrum's acid | 0 | E Parameter: -10.28 | J. Org. Chem. 2008, 73, 2738-2745 DOI: 10.1021/jo702590s | |
 2-benzylidene-indan-1,3-dione | 0 | E Parameter: -10.11 | Org. Biomol. Chem. 2007, 5, 3020-3026 DOI: 10.1039/b708025e | |
 benzylidenemalononitrile | 0 | E Parameter: -9.42 | J. Org. Chem. 2003, 68, 6880-6886 DOI: 10.1021/jo0344182 | |
 benzylidene-Meldrum's acid | 0 | E Parameter: -9.15 | J. Org. Chem. 2008, 73, 2738-2745 DOI: 10.1021/jo702590s |