
pop(tol)CH+ | 0 | E Parameter: 2.16
|    | Acc. Chem. Res. 2003, 36, 66-77
DOI: 10.1021/ar020094c |

fc(ani)CH+ | 0 | E Parameter: -2.90
|    | Acc. Chem. Res. 2003, 36, 66-77
DOI: 10.1021/ar020094c |

fc(Me)CH+ | 0 | E Parameter: -2.57
|  | Acc. Chem. Res. 2003, 36, 66-77
DOI: 10.1021/ar020094c |

fc2CH+ | 0 | E Parameter: -8.54
|    | Acc. Chem. Res. 2003, 36, 66-77
DOI: 10.1021/ar020094c |

C7H7+-Fe(CO)3 | | E Parameter: -3.49
|    | Acc. Chem. Res. 2003, 36, 66-77
DOI: 10.1021/ar020094c |

C7H9+-Fe(CO)3 | | E Parameter: -9.21
|    | Acc. Chem. Res. 2003, 36, 66-77
DOI: 10.1021/ar020094c |

C6H7+-Fe(CO)3 | | E Parameter: -7.76
|    | Acc. Chem. Res. 2003, 36, 66-77
DOI: 10.1021/ar020094c |

C6H6OMe+-Fe(CO)3 | | E Parameter: -8.94
|    | Acc. Chem. Res. 2003, 36, 66-77
DOI: 10.1021/ar020094c |

benzaldehyde-boron trichloride complex | | E Parameter: 1.12
|    | Acc. Chem. Res. 2003, 36, 66-77
DOI: 10.1021/ar020094c |

4-chlorobenzaldehyde-boron trichloride complex | | E Parameter: 1.44
|    | Acc. Chem. Res. 2003, 36, 66-77
DOI: 10.1021/ar020094c |

tropylium ion | 0 | E Parameter: -3.72
|    | Acc. Chem. Res. 2003, 36, 66-77
DOI: 10.1021/ar020094c |

propyn-1-ylium-Co2(CO)6 | | E Parameter: -0.84
|    | Acc. Chem. Res. 2003, 36, 66-77
DOI: 10.1021/ar020094c |

1-phenyl-proyn-ylium-Co2(CO)6 | | E Parameter: -0.97
|    | Acc. Chem. Res. 2003, 36, 66-77
DOI: 10.1021/ar020094c |

3-phenylpropyn-1-ylium-Co2(CO)6 | | E Parameter: -1.58
|    | Acc. Chem. Res. 2003, 36, 66-77
DOI: 10.1021/ar020094c |

3-(trimethylsilyl)propyn-1-ylium-Co2(CO)6 | | E Parameter: -1.60
|    | Acc. Chem. Res. 2003, 36, 66-77
DOI: 10.1021/ar020094c |

1-phenyl-propyn-1-ylium-Co2(CO)5(PPh3) | | E Parameter: -6.19
|    | Acc. Chem. Res. 2003, 36, 66-77
DOI: 10.1021/ar020094c |

2-phenyl-1,3-dithiolan-2-ylium ion | 0 | E Parameter: -5.91
|    | Acc. Chem. Res. 2003, 36, 66-77
DOI: 10.1021/ar020094c |

2-phenyl-1,3-dithianylium ion | 0 | E Parameter: -6.43
|    | Acc. Chem. Res. 2003, 36, 66-77
DOI: 10.1021/ar020094c |

1,3-dithianylium ion | 0 | E Parameter: -2.14
|    | Acc. Chem. Res. 2003, 36, 66-77
DOI: 10.1021/ar020094c |

N-ethylidenecarbazolium ion | 0 | E Parameter: 2.41
|    | Acc. Chem. Res. 2003, 36, 66-77
DOI: 10.1021/ar020094c |

N-methylacridinium ion | 0 | E Parameter: -7.15
|    | Acc. Chem. Res. 2003, 36, 66-77
DOI: 10.1021/ar020094c |

dimethylmethyleneammonium ion | 0 | E Parameter: -6.69
|  | Acc. Chem. Res. 2003, 36, 66-77
DOI: 10.1021/ar020094c |

methyl(phenyl)methyleneammonium ion | 0 | E Parameter: -5.17
|  | Acc. Chem. Res. 2003, 36, 66-77
DOI: 10.1021/ar020094c |

Vilsmeier ion | 0 | E Parameter: -5.77
|  | Acc. Chem. Res. 2003, 36, 66-77
DOI: 10.1021/ar020094c |

methoxy-phenylmethylium ion | 0 | E Parameter: 2.97
|    | Acc. Chem. Res. 2003, 36, 66-77
DOI: 10.1021/ar020094c |

methoxy-(4-methoxyphenyl)methylium ion | 0 | E Parameter: 0.14
|    | Acc. Chem. Res. 2003, 36, 66-77
DOI: 10.1021/ar020094c |

methoxy-(4-methylphenyl)methylium ion | 0 | E Parameter: 1.90
|    | Acc. Chem. Res. 2003, 36, 66-77
DOI: 10.1021/ar020094c |

Pd-allyl cation | | E Parameter: -10.11
|    | Acc. Chem. Res. 2003, 36, 66-77
DOI: 10.1021/ar020094c |

flavylium ion | 0 | E Parameter: -3.45
|    | Acc. Chem. Res. 2003, 36, 66-77
DOI: 10.1021/ar020094c |

2-(4-methoxyphenyl)chromenylium ion | 0 | E Parameter: -4.95
|    | Acc. Chem. Res. 2003, 36, 66-77
DOI: 10.1021/ar020094c |

1,1,3-triphenylallylium ion | 0 | E Parameter: 0.98
|    | Acc. Chem. Res. 2003, 36, 66-77
DOI: 10.1021/ar020094c |

1,1-dianisyl-3-phenylallylium ion | 0 | E Parameter: -2.67
|    | Acc. Chem. Res. 2003, 36, 66-77
DOI: 10.1021/ar020094c |

1,1-bis(4-dimethylaminophenyl)-3-phenylallylium ion | 0 | E Parameter: -8.97
|    | Acc. Chem. Res. 2003, 36, 66-77
DOI: 10.1021/ar020094c |

1,1,3-tris(4-(dimethylamino)phenyl)allylium ion | 0 | E Parameter: -9.84
|    | Acc. Chem. Res. 2003, 36, 66-77
DOI: 10.1021/ar020094c |

1-phenylcyclopent-2-enylium ion | 0 | E Parameter: 2.89
|    | Acc. Chem. Res. 2003, 36, 66-77
DOI: 10.1021/ar020094c |

1-(4-chlorophenyl)cyclopent-2-enylium ion | 0 | E Parameter: 3.20
|    | Acc. Chem. Res. 2003, 36, 66-77
DOI: 10.1021/ar020094c |

1,3-diphenylpropyn-1-ylium-Cr(CO)3 | | E Parameter: 1.07
|    | Acc. Chem. Res. 2003, 36, 66-77
DOI: 10.1021/ar020094c |

1,1,3-triphenylpropyn-1-ylium-Cr(CO)3 | | E Parameter: -0.25
|    | Acc. Chem. Res. 2003, 36, 66-77
DOI: 10.1021/ar020094c |

ani(Br)2QM | 0 | E Parameter: -8.63
|     | Angew. Chem. Int. Ed. 2002, 41, 91-95
DOI: 10.1002/1521-3773(20020104)41:1<91::AID-ANIE91>3.0.CO;2-P |

ani(Ph)2QM | 0 | E Parameter: -12.18
|     | Angew. Chem. Int. Ed. 2002, 41, 91-95
DOI: 10.1002/1521-3773(20020104)41:1<91::AID-ANIE91>3.0.CO;2-P |

dma(Ph)2QM | 0 | E Parameter: -13.39
|     | Angew. Chem. Int. Ed. 2002, 41, 91-95
DOI: 10.1002/1521-3773(20020104)41:1<91::AID-ANIE91>3.0.CO;2-P |

tol(t-Bu)2QM | 0 | E Parameter: -15.83
|     | Angew. Chem. Int. Ed. 2002, 41, 91-95
DOI: 10.1002/1521-3773(20020104)41:1<91::AID-ANIE91>3.0.CO;2-P |

ani(t-Bu)2QM | | E Parameter: -16.11
|     | Angew. Chem. Int. Ed. 2002, 41, 91-95
DOI: 10.1002/1521-3773(20020104)41:1<91::AID-ANIE91>3.0.CO;2-P |

dma(t-Bu)2QM | | E Parameter: -17.29
|     | Angew. Chem. Int. Ed. 2002, 41, 91-95
DOI: 10.1002/1521-3773(20020104)41:1<91::AID-ANIE91>3.0.CO;2-P |

jul(t-Bu)2QM | 0 | E Parameter: -17.90
|     | Angew. Chem. Int. Ed. 2002, 41, 91-95
DOI: 10.1002/1521-3773(20020104)41:1<91::AID-ANIE91>3.0.CO;2-P |

Me2N+=CH-CH-Ph | 0 | E Parameter: -9.20
|    | Angew. Chem. Int. Ed. 2008, 47, 8723-8726
DOI: 10.1002/anie.200802889 |

pyrN+=CH-CH-Ph | 0 | E Parameter: -9.80
|    | Angew. Chem. Int. Ed. 2008, 47, 8723-8726
DOI: 10.1002/anie.200802889 |

pipN+=CH-CH-Ph | 0 | E Parameter: -10.30
|  | Angew. Chem. Int. Ed. 2008, 47, 8723-8726
DOI: 10.1002/anie.200802889 |

(PhCH2)2N+=CH-CH-Ph | 0 | E Parameter: -8.50
|    | Angew. Chem. Int. Ed. 2008, 47, 8723-8726
DOI: 10.1002/anie.200802889 |

Jorgensen/Hayashi-iminium ion | 0 | E Parameter: -8.20
|    | Angew. Chem. Int. Ed. 2008, 47, 8723-8726
DOI: 10.1002/anie.200802889 |

MacMillan-iminium ion (1st generation) | MeCN | E Parameter: -7.37
|    | Angew. Chem. Int. Ed. 2011, 50, 9953-9956
DOI: 10.1002/anie.201103683 |

mor iminium | 0 | E Parameter: -8.60
|    | Angew. Chem. Int. Ed. 2008, 47, 8723-8726
DOI: 10.1002/anie.200802889 |

MacMillan-iminium ion (2nd generation) | MeCN | E Parameter: -5.52
|    | Angew. Chem. Int. Ed. 2011, 50, 9953-9956
DOI: 10.1002/anie.201103683 |

MacMillan-iminium ion (spiro) | MeCN | E Parameter: -7.67
|    | Angew. Chem. Int. Ed. 2011, 50, 9953-9956
DOI: 10.1002/anie.201103683 |

indolecarboxylate derived iminium ion | dichloromethane | E Parameter: -9.50
|   | Angew. Chem. Int. Ed. 2009, 48, 5034-5037
DOI: 10.1002/anie.200900933 |

2-cinnamoyl-1,3-dimethyl-1H-imidazol-3-ium | DMSO | E Parameter: -11.52
|    | Angew. Chem. Int. Ed. 2012, 51, 5234-5238
DOI: 10.1002/anie.201109042 |

(S,E)-2,2,3-trimethyl-4-oxo-5-((perfluorophenyl)methyl)-1-((E)-3-phenylallylidene)imidazolidin-1-ium | dichloromethane | E Parameter: -6.00
|   | Angew. Chem. Int. Ed. 2013, 52, 7967-7971
DOI: 10.1002/anie.201301864 |

(S,E)-2,2,3-trimethyl-4-oxo-1-((E)-3-phenylallylidene)-5-(3,4,5-trimethoxybenzyl)imidazolidin-1-ium | dichloromethane | E Parameter: -7.00
|   | Angew. Chem. Int. Ed. 2013, 52, 7967-7971
DOI: 10.1002/anie.201301864 |

ethenesulfonyl fluoride (ESF) | DMSO | E Parameter: -12.09
|    | Angew. Chem. Int. Ed. 2016, 55, 12664-12667
DOI: 10.1002/anie.201601875 |

2-phenylethene-1-sulfonyl fluoride | DMSO | E Parameter: -16.63
|    | Angew. Chem. Int. Ed. 2016, 55, 12664-12667
DOI: 10.1002/anie.201601875 |

N-(phenylsulfonyl)-N-((trifluoromethyl)thio)benzenesulfonamide | 0 | E Parameter: -6.06
|    | Angew. Chem. Int. Ed. 2018, 57, 12690-12695
DOI: 10.1002/anie.201805859 |

N-(trifluoromethyl)thio)phthalimide | 0 | E Parameter: -11.92
|    | Angew. Chem. Int. Ed. 2018, 57, 12690-12695
DOI: 10.1002/anie.201805859 |

N-(trifluoromethyl)thio)saccharin | 0 | E Parameter: -6.48
|    | Angew. Chem. Int. Ed. 2018, 57, 12690-12695
DOI: 10.1002/anie.201805859 |

N-(trifluoromethyl)thio)succinimide | 0 | E Parameter: -12.56
|    | Angew. Chem. Int. Ed. 2018, 57, 12690-12695
DOI: 10.1002/anie.201805859 |

N-(difluoromethyl)thio)phthalimide | 0 | E Parameter: -11.86
|    | Angew. Chem. Int. Ed. 2018, 57, 12690-12695
DOI: 10.1002/anie.201805859 |

N-methyl-N-((trifluoromethyl)thio)-p-toluenesulfonamide | 0 | E Parameter: -13.44
|    | Angew. Chem. Int. Ed. 2018, 57, 12690-12695
DOI: 10.1002/anie.201805859 |

N-methyl-N-((trifluoromethyl)thio)-aniline | 0 | E Parameter: -23.32
|  | Angew. Chem. Int. Ed. 2018, 57, 12690-12695
DOI: 10.1002/anie.201805859 |

((2-phenylpropan-2-yl)oxy)(trifluoromethyl)sulfane | 0 | E Parameter: -13.77
|    | Angew. Chem. Int. Ed. 2018, 57, 12690-12695
DOI: 10.1002/anie.201805859 |

((2-(2-iodophenyl)propan-2-yl)oxy)(trifluoromethyl)sulfane | 0 | E Parameter: -14.52
|    | Angew. Chem. Int. Ed. 2018, 57, 12690-12695
DOI: 10.1002/anie.201805859 |

4-phenylbut-3-yn-2-one | 0 | E Parameter: -16.90
|  | Angew. Chem. Int. Ed. 2019, 58, 17704-17708
DOI: 10.1002/anie.201909803 |

hex-3-yn-2-one | 0 | E Parameter: -17.90
|  | Angew. Chem. Int. Ed. 2019, 58, 17704-17708
DOI: 10.1002/anie.201909803 |

butynone | 0 | E Parameter: -16.60
|  | Angew. Chem. Int. Ed. 2019, 58, 17704-17708
DOI: 10.1002/anie.201909803 |

(S,E)-5-benzyl-2,2,3-trimethyl-1-((E)-3-(4-nitrophenyl)allylidene)-4-oxoimidazolidin-1-ium | dichloromethane | E Parameter: -5.90
|   | Asian J. Org. Chem. 2014, 3, 550-555
DOI: 10.1002/ajoc.201402009 |

(S,E)-5-benzyl-1-((E)-3-(4-cyanophenyl)allylidene)-2,2,3-trimethyl-4-oxoimidazolidin-1-ium | dichloromethane | E Parameter: -6.00
|   | Asian J. Org. Chem. 2014, 3, 550-555
DOI: 10.1002/ajoc.201402009 |

(S,E)-5-benzyl-1-((E)-3-(4-(dimethylamino)phenyl)allylidene)-2,2,3-trimethyl-4-oxoimidazolidin-1-ium | dichloromethane | E Parameter: -10.60
|   | Asian J. Org. Chem. 2014, 3, 550-555
DOI: 10.1002/ajoc.201402009 |

(S,E)-5-benzyl-2,2,3-trimethyl-4-oxo-1-((E)-3-(p-tolyl)allylidene)imidazolidin-1-ium | dichloromethane | E Parameter: -7.20
|   | Asian J. Org. Chem. 2014, 3, 550-555
DOI: 10.1002/ajoc.201402009 |

(S,E)-5-benzyl-1-((E)-3-(4-methoxyphenyl)allylidene)-2,2,3-trimethyl-4-oxoimidazolidin-1-ium | dichloromethane | E Parameter: -8.00
|   | Asian J. Org. Chem. 2014, 3, 550-555
DOI: 10.1002/ajoc.201402009 |

(S,E)-5-benzyl-2,2,3-trimethyl-1-((E)-3-(3-nitrophenyl)allylidene)-4-oxoimidazolidin-1-ium | dichloromethane | E Parameter: -6.10
|   | Asian J. Org. Chem. 2014, 3, 550-555
DOI: 10.1002/ajoc.201402009 |

(S,E)-5-((1H-indol-3-yl)methyl)-2,2,3-trimethyl-4-oxo-1-((E)-3-phenylallylidene)imidazolidin-1-ium | dichloromethane | E Parameter: -7.30
|   | Asian J. Org. Chem. 2014, 3, 550-555
DOI: 10.1002/ajoc.201402009 |

(2S,5S)-5-benzyl-3-methyl-4-oxo-1-(3-phenylallylidene)-2-((E)-styryl)imidazolidin-1-ium | dichloromethane | E Parameter: -5.90
|   | Asian J. Org. Chem. 2014, 3, 550-555
DOI: 10.1002/ajoc.201402009 |

(ethene-1,1-diyldisulfonyl)dibenzene | DMSO | E Parameter: -7.50
|    | Chem. Asian J. 2012, 7, 1401-1407
DOI: 10.1002/asia.201101046 |

(2-phenylethene-1,1-diyldisulfonyl)dibenzene | DMSO | E Parameter: -12.93
|    | Chem. Asian J. 2012, 7, 1401-1407
DOI: 10.1002/asia.201101046 |

(2-(4-methoxyphenyl)ethene-1,1-diyldisulfonyl)dibenzene | DMSO | E Parameter: -13.88
|    | Chem. Asian J. 2012, 7, 1401-1407
DOI: 10.1002/asia.201101046 |

4-(2,2-bis(phenylsulfonyl)vinyl)-N,N-dimethylaniline | DMSO | E Parameter: -16.53
|    | Chem. Asian J. 2012, 7, 1401-1407
DOI: 10.1002/asia.201101046 |

2-benzylidenebenzo[d][1,3]dithiole 1,1,3,3-tetraoxide | DMSO | E Parameter: -11.78
|    | Chem. Asian J. 2012, 7, 1401-1407
DOI: 10.1002/asia.201101046 |

2-(4-methoxybenzylidene)benzo[d][1,3]dithiole 1,1,3,3-tetraoxide | DMSO | E Parameter: -13.02
|    | Chem. Asian J. 2012, 7, 1401-1407
DOI: 10.1002/asia.201101046 |

2-(4-methoxybenzylidene)-1,3-dithiane 1,1,3,3-tetraoxide | DMSO | E Parameter: -14.55
|    | Chem. Asian J. 2012, 7, 1401-1407
DOI: 10.1002/asia.201101046 |

ethyl 3-nitro-1H-indole-1-carboxylate | 0 | E Parameter: -14.10
|   | Chem. Commun. 2021, 57, 10071-10074
DOI: 10.1039/d1cc04074j |

3-nitro-1-tosyl-1H-indole | 0 | E Parameter: -14.87
|    | Chem. Commun. 2021, 57, 10071-10074
DOI: 10.1039/d1cc04074j |

4-bromo-3-nitro-1-tosyl-1H-indole | 0 | E Parameter: -14.60
|   | Chem. Commun. 2021, 57, 10071-10074
DOI: 10.1039/d1cc04074j |

3,6-dinitro-1-tosyl-1H-indole | 0 | E Parameter: -12.90
|    | Chem. Commun. 2021, 57, 10071-10074
DOI: 10.1039/d1cc04074j |

4-nitrobenzodifuroxan (NBDF) | 0 | E Parameter: -6.15
|    | Chem. Eur. J. 2007, 13, 8317-8324
DOI: 10.1002/chem.200700676 |

diethyl benzylidene malonate | 0 | E Parameter: -20.55
|    | Chem. Eur. J. 2008, 14, 9675-9682
DOI: 10.1002/chem.200801277 |

diethyl 4-nitrobenzylidene malonate | 0 | E Parameter: -17.67
|    | Chem. Eur. J. 2008, 14, 9675-9682
DOI: 10.1002/chem.200801277 |

diethyl 4-cyano-benzylidene malonate | 0 | E Parameter: -18.06
|    | Chem. Eur. J. 2008, 14, 9675-9682
DOI: 10.1002/chem.200801277 |

diethyl 3-chloro-benzylidene malonate | 0 | E Parameter: -18.98
|    | Chem. Eur. J. 2008, 14, 9675-9682
DOI: 10.1002/chem.200801277 |

diethyl 4-methyl-benzylidene malonate | 0 | E Parameter: -21.11
|    | Chem. Eur. J. 2008, 14, 9675-9682
DOI: 10.1002/chem.200801277 |

diethyl 4-methoxy-benzylidene malonate | 0 | E Parameter: -21.47
|    | Chem. Eur. J. 2008, 14, 9675-9682
DOI: 10.1002/chem.200801277 |

diethyl 4-(dimethylamino)benzylidene malonate | 0 | E Parameter: -23.10
|    | Chem. Eur. J. 2008, 14, 9675-9682
DOI: 10.1002/chem.200801277 |

diethyl 2-((1-methyl-1,2,3,4-tetrahydroquinolin-6-yl)methylene)malonate | 0 | E Parameter: -23.40
|  | Chem. Eur. J. 2008, 14, 9675-9682
DOI: 10.1002/chem.200801277 |

diethyl 2-((julolidine-9-yl)methylene)malonate | 0 | E Parameter: -23.80
|  | Chem. Eur. J. 2008, 14, 9675-9682
DOI: 10.1002/chem.200801277 |

bh6a | | E Parameter: -18.60
|   | Chem. Eur. J. 2010, 16, 1365-1371
DOI: 10.1002/chem.200902487 |

bh 6f | | E Parameter: -18.90
|   | Chem. Eur. J. 2010, 16, 1365-1371
DOI: 10.1002/chem.200902487 |

bh 6i | | E Parameter: -19.10
|   | Chem. Eur. J. 2010, 16, 1365-1371
DOI: 10.1002/chem.200902487 |

bh6j | | E Parameter: -17.90
|   | Chem. Eur. J. 2010, 16, 1365-1371
DOI: 10.1002/chem.200902487 |

bh 6k | | E Parameter: -18.20
|  | Chem. Eur. J. 2010, 16, 1365-1371
DOI: 10.1002/chem.200902487 |

bh 6l | | E Parameter: -18.90
|  | Chem. Eur. J. 2010, 16, 1365-1371
DOI: 10.1002/chem.200902487 |

bh 6m | | E Parameter: -17.30
|  | Chem. Eur. J. 2010, 16, 1365-1371
DOI: 10.1002/chem.200902487 |

1,2-diaza-1,3-diene 1a | DMSO | E Parameter: -13.28
|    | Chem. Eur. J. 2010, 16, 12008-12016
DOI: 10.1002/chem.201000828 |

1,2-diaza-1,3-diene 1b | DMSO | E Parameter: -13.90
|    | Chem. Eur. J. 2010, 16, 12008-12016
DOI: 10.1002/chem.201000828 |

1,2-diaza-1,3-diene 1c | DMSO | E Parameter: -14.91
|    | Chem. Eur. J. 2010, 16, 12008-12016
DOI: 10.1002/chem.201000828 |

1,2-diaza-1,3-diene 1d | DMSO | E Parameter: -15.38
|    | Chem. Eur. J. 2010, 16, 12008-12016
DOI: 10.1002/chem.201000828 |

1,2-diaza-1,3-diene 1e | MeOH | E Parameter: -14.90
|  | Chem. Eur. J. 2010, 16, 12008-12016
DOI: 10.1002/chem.201000828 |

1,2-diaza-1,3-diene 1f | MeOH | E Parameter: -15.30
|  | Chem. Eur. J. 2010, 16, 12008-12016
DOI: 10.1002/chem.201000828 |

dibenzyl azodicarboxylate | MeCN | E Parameter: -8.89
|    | Chem. Eur. J. 2010, 16, 11670-11677
DOI: 10.1002/chem.201001598 |

diethyl azodicarboxylate | MeCN | E Parameter: -10.15
|    | Chem. Eur. J. 2010, 16, 11670-11677
DOI: 10.1002/chem.201001598 |

diisopropyl azodicarboxylate | MeCN | E Parameter: -10.71
|    | Chem. Eur. J. 2010, 16, 11670-11677
DOI: 10.1002/chem.201001598 |

di-tert-butyl azodicarboxylate | MeCN | E Parameter: -12.23
|    | Chem. Eur. J. 2010, 16, 11670-11677
DOI: 10.1002/chem.201001598 |

9-phenyl-9H-fluoren-9-ylium | dichloromethane | E Parameter: 2.41
|    | Chem. Eur. J. 2017, 23, 623-630
DOI: 10.1002/chem.201603963 |

9-(4-methoxyphenyl)-9H-fluoren-9-ylium | dichloromethane | E Parameter: 0.85
|    | Chem. Eur. J. 2017, 23, 623-630
DOI: 10.1002/chem.201603963 |

9-(4-(dimethylamino)phenyl)-9H-fluoren-9-ylium | dichloromethane | E Parameter: -4.51
|    | Chem. Eur. J. 2017, 23, 623-630
DOI: 10.1002/chem.201603963 |

methyl diazoacetate | 0 | E Parameter: -18.50
|    | Chem. Eur. J. 2022, 28, e202201376
DOI: 10.1002/chem.202201376 |

dimethyl diazomalonate | 0 | E Parameter: -18.20
|    | Chem. Eur. J. 2022, 28, e202201376
DOI: 10.1002/chem.202201376 |

(p-nitrophenyl)diazomethane | 0 | E Parameter: -18.30
|    | Chem. Eur. J. 2022, 28, e202201376
DOI: 10.1002/chem.202201376 |

diphenyldiazomethane | 0 | E Parameter: -21.40
|  | Chem. Eur. J. 2022, 28, e202201376
DOI: 10.1002/chem.202201376 |
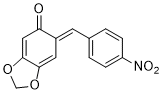
(E)-6-(4-nitrobenzylidene)benzo[d][1,3]dioxol-5(6H)-one | 0 | E Parameter: -13.15
|    | Chem. Eur. J. 2025, 31, e202403785
DOI: 10.1002/chem.202403785 |
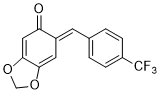
(E)-6-(4-(trifluoromethyl)benzylidene)benzo[d][1,3]dioxol-5(6H)-one | 0 | E Parameter: -13.54
|    | Chem. Eur. J. 2025, 31, e202403785
DOI: 10.1002/chem.202403785 |
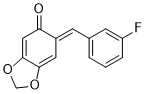
(E)-6-(3-fluorobenzylidene)benzo[d][1,3]dioxol-5(6H)-one | 0 | E Parameter: -14.00
|    | Chem. Eur. J. 2025, 31, e202403785
DOI: 10.1002/chem.202403785 |
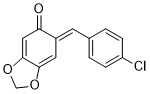
(E)-6-(4-chlorobenzylidene)benzo[d][1,3]dioxol-5(6H)-one | 0 | E Parameter: -13.99
|    | Chem. Eur. J. 2025, 31, e202403785
DOI: 10.1002/chem.202403785 |
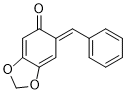
(E)-6-benzylidenebenzo[d][1,3]dioxol-5(6H)-one | 0 | E Parameter: -14.47
|    | Chem. Eur. J. 2025, 31, e202403785
DOI: 10.1002/chem.202403785 |
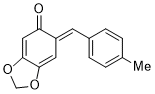
(E)-6-(4-methylbenzylidene)benzo[d][1,3]dioxol-5(6H)-one | 0 | E Parameter: -14.85
|    | Chem. Eur. J. 2025, 31, e202403785
DOI: 10.1002/chem.202403785 |
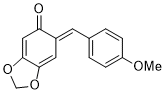
(E)-6-(4-methoxybenzylidene)benzo[d][1,3]dioxol-5(6H)-one | 0 | E Parameter: -15.20
|    | Chem. Eur. J. 2025, 31, e202403785
DOI: 10.1002/chem.202403785 |
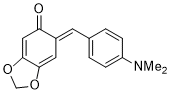
(E)-6-(4-(dimethylamino)benzylidene)benzo[d][1,3]dioxol-5(6H)-one | 0 | E Parameter: -16.07
|    | Chem. Eur. J. 2025, 31, e202403785
DOI: 10.1002/chem.202403785 |
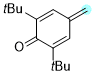
2,6-di-tert-butyl-4-methylenecyclohexa-2,5-dien-1-one | 0 | E Parameter: -11.17
|    | Chem. Eur. J. 2025, 31, e202501224
DOI: 10.1002/chem.202501224 |
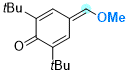
2,6-di-tert-butyl-4-(methoxymethylene)cyclohexa-2,5-dien-1-one | 0 | E Parameter: -15.10
|    | Chem. Eur. J. 2025, 31, e202501224
DOI: 10.1002/chem.202501224 |
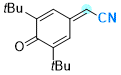
2-(3,5-di-tert-butyl-4-oxocyclohexa-2,5-dien-1-ylidene)acetonitrile | 0 | E Parameter: -11.88
|    | Chem. Eur. J. 2025, 31, e202501224
DOI: 10.1002/chem.202501224 |
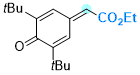
ethyl 2-(3,5-di-tert-butyl-4-oxocyclohexa-2,5-dien-1-ylidene)acetate | 0 | E Parameter: -12.01
|    | Chem. Eur. J. 2025, 31, e202501224
DOI: 10.1002/chem.202501224 |
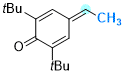
2,6-di-tert-butyl-4-ethylidenecyclohexa-2,5-dien-1-one | 0 | E Parameter: -13.68
|    | Chem. Eur. J. 2025, 31, e202501224
DOI: 10.1002/chem.202501224 |
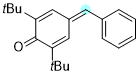
4-benzylidene-2,6-di-tert-butylcyclohexa-2,5-dien-1-one | 0 | E Parameter: -15.58
|    | Chem. Eur. J. 2025, 31, e202501224
DOI: 10.1002/chem.202501224 |

3-methylcyclopentenone | DMSO | E Parameter: -28.90
|  | Chem. Sci. 2021, 12, 4850-4865
DOI: 10.1039/D0SC06628A |

2-methylcyclopentenone | DMSO | E Parameter: -22.10
|    | Chem. Sci. 2021, 12, 4850-4865
DOI: 10.1039/D0SC06628A |

cyclopentenone | DMSO | E Parameter: -20.60
|    | Chem. Sci. 2021, 12, 4850-4865
DOI: 10.1039/D0SC06628A |

furan-2(5H)-one | DMSO | E Parameter: -20.70
|    | Chem. Sci. 2021, 12, 4850-4865
DOI: 10.1039/D0SC06628A |

2-methylcyclohexenone | DMSO | E Parameter: -27.50
|  | Chem. Sci. 2021, 12, 4850-4865
DOI: 10.1039/D0SC06628A |

cyclohexenone | DMSO | E Parameter: -22.10
|    | Chem. Sci. 2021, 12, 4850-4865
DOI: 10.1039/D0SC06628A |

dihydro-2H-pyran-2-one | DMSO | E Parameter: -21.80
|   | Chem. Sci. 2021, 12, 4850-4865
DOI: 10.1039/D0SC06628A |

3-methylenetetrahydropyran-2-one | DMSO | E Parameter: -19.50
|    | Chem. Sci. 2021, 12, 4850-4865
DOI: 10.1039/D0SC06628A |

3-methylenedihydrofuranone | DMSO | E Parameter: -19.40
|    | Chem. Sci. 2021, 12, 4850-4865
DOI: 10.1039/D0SC06628A |

3-methylcyclohexenone | DMSO | E Parameter: -29.60
|  | Chem. Sci. 2021, 12, 4850-4865
DOI: 10.1039/D0SC06628A |

cycloheptenone | DMSO | E Parameter: -22.00
|    | Chem. Sci. 2021, 12, 4850-4865
DOI: 10.1039/D0SC06628A |

2,6-diphenyl-4-benzylidenecyclohexa-2,5-dienone | 0 | E Parameter: -11.87
|    | Eur. J. Org. Chem. 2009, , 3203-3211
DOI: 10.1002/ejoc.200900299 |

2,6-dimethoxy-4-(4-methoxybenzylidene)cyclohexa-2,5-dienone | 0 | E Parameter: -16.38
|    | Eur. J. Org. Chem. 2009, , 3203-3211
DOI: 10.1002/ejoc.200900299 |

2,6-dimethoxy-4-(4-(dimethylamino)benzylidene)cyclohexa-2,5-dienone | 0 | E Parameter: -17.18
|    | Eur. J. Org. Chem. 2009, , 3203-3211
DOI: 10.1002/ejoc.200900299 |

2,6-dimethyl-4-(4-(dimethylamino)benzylidene)cyclohexa-2,5-dienone | 0 | E Parameter: -16.36
|    | Eur. J. Org. Chem. 2009, , 3203-3211
DOI: 10.1002/ejoc.200900299 |

2,6-di-tert.butyl-4-(3-fluorobenzylidene)cyclohexa-2,5-dienone | 0 | E Parameter: -15.03
|    | Eur. J. Org. Chem. 2009, , 3203-3211
DOI: 10.1002/ejoc.200900299 |

2,6-di-tert.butyl-4-(3,5-difluorobenzylidene)cyclohexa-2,5-dienone | 0 | E Parameter: -14.50
|    | Eur. J. Org. Chem. 2009, , 3203-3211
DOI: 10.1002/ejoc.200900299 |

2,6-di-tert.butyl-4-(4-nitrobenzylidene)cyclohexa-2,5-dienone | 0 | E Parameter: -14.36
|    | Eur. J. Org. Chem. 2009, , 3203-3211
DOI: 10.1002/ejoc.200900299 |

(4-MeO)-tritylium ion | | E Parameter: -1.59
|  | Eur. J. Org. Chem. 2011, , 6470-6475
DOI: 10.1002/ejoc.201100910 |

(4-F)-tritylium ion | | E Parameter: 0.35
|  | Eur. J. Org. Chem. 2011, , 6470-6475
DOI: 10.1002/ejoc.201100910 |

(4-F)2-tritylium ion | | E Parameter: 0.17
|  | Eur. J. Org. Chem. 2011, , 6470-6475
DOI: 10.1002/ejoc.201100910 |

(4-F)3-tritylium ion | | E Parameter: 0.05
|  | Eur. J. Org. Chem. 2011, , 6470-6475
DOI: 10.1002/ejoc.201100910 |

(3-F)-tritylium ion | | E Parameter: 1.01
|  | Eur. J. Org. Chem. 2011, , 6470-6475
DOI: 10.1002/ejoc.201100910 |

(3-F)2-tritylium ion | | E Parameter: 1.54
|  | Eur. J. Org. Chem. 2011, , 6470-6475
DOI: 10.1002/ejoc.201100910 |

(3-F)3-tritylium ion | | E Parameter: 2.07
|  | Eur. J. Org. Chem. 2011, , 6470-6475
DOI: 10.1002/ejoc.201100910 |

(3-F)4-tritylium ion | | E Parameter: 2.54
|  | Eur. J. Org. Chem. 2011, , 6470-6475
DOI: 10.1002/ejoc.201100910 |

maleic anhydride | DMSO | E Parameter: -11.31
|    | Eur. J. Org. Chem. 2014, , 2956-2963
DOI: 10.1002/ejoc.201301779 |

N-methyl maleimide | DMSO | E Parameter: -14.07
|    | Eur. J. Org. Chem. 2014, , 2956-2963
DOI: 10.1002/ejoc.201301779 |

fumaronitrile | DMSO | E Parameter: -15.71
|    | Eur. J. Org. Chem. 2014, , 2956-2963
DOI: 10.1002/ejoc.201301779 |

diethyl fumarate | DMSO | E Parameter: -17.79
|    | Eur. J. Org. Chem. 2014, , 2956-2963
DOI: 10.1002/ejoc.201301779 |

diethyl maleate | DMSO | E Parameter: -19.49
|    | Eur. J. Org. Chem. 2014, , 2956-2963
DOI: 10.1002/ejoc.201301779 |

(E)-2-(tert-butyl)-3-(4-methylbenzylidene)-3H-indol-1-ium | dichloromethane | E Parameter: -5.06
|  | Eur. J. Org. Chem. 2016, , 4050-4058
DOI: 10.1002/ejoc.201600572 |

2,6-di-tert-butyl-4-(2,2,2-trifluoroethylidene)cyclohexa-2,5-dien-1-one | DMSO | E Parameter: -11.68
|    | Eur. J. Org. Chem. 2020, , 3812-3817
DOI: 10.1002/ejoc.202000295 |

Umemoto I (triflate) | 0 | E Parameter: -13.08
|    | Eur. J. Org. Chem. 2024, 27, e202400085
DOI: 10.1002/ejoc.202400085 |

Umemoto I (tetrafluoroborate) | 0 | E Parameter: -13.39
|    | Eur. J. Org. Chem. 2024, 27, e202400085
DOI: 10.1002/ejoc.202400085 |

Umemoto II (triflate) | 0 | E Parameter: -12.80
|    | Eur. J. Org. Chem. 2024, 27, e202400085
DOI: 10.1002/ejoc.202400085 |

dicarbonyl(cyclopentadienyl)propene-iron(II) | | E Parameter: -11.20
|    | Helv. Chim. Acta 2005, 88, 1754-1768
DOI: 10.1002/hlca.200590137 |

benzhydrylium ion Ph2CH+ | 0 | E Parameter: 5.47
|      | J. Am. Chem. Soc. 2012, 134, 13902-13911
DOI: 10.1021/ja306522b |

(pcp)2CH+ | 0 | E Parameter: 5.48
|      | J. Am. Chem. Soc. 2012, 134, 13902-13911
DOI: 10.1021/ja306522b |

pfp(Ph)CH+ | 0 | E Parameter: 5.20
|      | J. Am. Chem. Soc. 2012, 134, 13902-13911
DOI: 10.1021/ja306522b |

(fur)2CH+ | 0 | E Parameter: -1.36
|      | J. Am. Chem. Soc. 2001, 123, 9500-9512
DOI: 10.1021/ja010890y |

(ani)2CH+ | 0 | E Parameter: 0.00
|      | J. Am. Chem. Soc. 2001, 123, 9500-9512
DOI: 10.1021/ja010890y |

(tol)2CH+ | 0 | E Parameter: 3.63
|      | J. Am. Chem. Soc. 2001, 123, 9500-9512
DOI: 10.1021/ja010890y |

(pfa)2CH+ | 0 | E Parameter: -3.14
|      | J. Am. Chem. Soc. 2001, 123, 9500-9512
DOI: 10.1021/ja010890y |

(mfa)2CH+ | 0 | E Parameter: -3.85
|      | J. Am. Chem. Soc. 2001, 123, 9500-9512
DOI: 10.1021/ja010890y |

(dpa)2CH+ | 0 | E Parameter: -4.72
|      | J. Am. Chem. Soc. 2001, 123, 9500-9512
DOI: 10.1021/ja010890y |

(mor)2CH+ | 0 | E Parameter: -5.53
|      | J. Am. Chem. Soc. 2001, 123, 9500-9512
DOI: 10.1021/ja010890y |

(mpa)2CH+ | 0 | E Parameter: -5.89
|      | J. Am. Chem. Soc. 2001, 123, 9500-9512
DOI: 10.1021/ja010890y |

(dma)2CH+ | 0 | E Parameter: -7.02
|      | J. Am. Chem. Soc. 2001, 123, 9500-9512
DOI: 10.1021/ja010890y |

(pyr)2CH+ | 0 | E Parameter: -7.69
|      | J. Am. Chem. Soc. 2001, 123, 9500-9512
DOI: 10.1021/ja010890y |

(thq)2CH+ | 0 | E Parameter: -8.22
|      | J. Am. Chem. Soc. 2001, 123, 9500-9512
DOI: 10.1021/ja010890y |

(ind)2CH+ | 0 | E Parameter: -8.76
|      | J. Am. Chem. Soc. 2001, 123, 9500-9512
DOI: 10.1021/ja010890y |

(jul)2CH+ | 0 | E Parameter: -9.45
|      | J. Am. Chem. Soc. 2001, 123, 9500-9512
DOI: 10.1021/ja010890y |

(lil)2CH+ | 0 | E Parameter: -10.04
|      | J. Am. Chem. Soc. 2001, 123, 9500-9512
DOI: 10.1021/ja010890y |

ani(pop)CH+ | 0 | E Parameter: 0.61
|      | J. Am. Chem. Soc. 2001, 123, 9500-9512
DOI: 10.1021/ja010890y |

ani(Ph)CH+ | 0 | E Parameter: 2.11
|      | J. Am. Chem. Soc. 2001, 123, 9500-9512
DOI: 10.1021/ja010890y |

ani(tol)CH+ | 0 | E Parameter: 1.48
|      | J. Am. Chem. Soc. 2001, 123, 9500-9512
DOI: 10.1021/ja010890y |

pop(Ph)CH+ | 0 | E Parameter: 2.90
|      | J. Am. Chem. Soc. 2001, 123, 9500-9512
DOI: 10.1021/ja010890y |

tol(Ph)CH+ | 0 | E Parameter: 4.43
|      | J. Am. Chem. Soc. 2012, 134, 13902-13911
DOI: 10.1021/ja306522b |

(pfp)2CH+ | 0 | E Parameter: 5.01
|      | J. Am. Chem. Soc. 2012, 134, 13902-13911
DOI: 10.1021/ja306522b |

fc(Ph)CH+ | 0 | E Parameter: -2.64
|      | J. Am. Chem. Soc. 2001, 123, 9500-9512
DOI: 10.1021/ja010890y |

tritylium ion (Ph3C+) | 0 | E Parameter: 0.51
|  | J. Am. Chem. Soc. 2003, 125, 286-295
DOI: 10.1021/ja021010y |

(4-CF3)2-tritylium ion | | E Parameter: 2.28
|  | J. Am. Chem. Soc. 2003, 125, 286-295
DOI: 10.1021/ja021010y |

(3-Cl)3-tritylium ion | | E Parameter: 1.99
|  | J. Am. Chem. Soc. 2003, 125, 286-295
DOI: 10.1021/ja021010y |

(4-CF3)-tritylium ion | | E Parameter: 1.33
|  | J. Am. Chem. Soc. 2003, 125, 286-295
DOI: 10.1021/ja021010y |

(3-Cl)-tritylium ion | | E Parameter: 1.06
|  | J. Am. Chem. Soc. 2003, 125, 286-295
DOI: 10.1021/ja021010y |

(3-CF3)-tritylium ion | | E Parameter: 1.18
|  | J. Am. Chem. Soc. 2003, 125, 286-295
DOI: 10.1021/ja021010y |

(4-Me)-tritylium ion | | E Parameter: -0.13
|  | J. Am. Chem. Soc. 2003, 125, 286-295
DOI: 10.1021/ja021010y |

(4-Me)2-tritylium ion | | E Parameter: -0.70
|  | J. Am. Chem. Soc. 2003, 125, 286-295
DOI: 10.1021/ja021010y |

(4-Me)3-tritylium ion | | E Parameter: -1.21
|  | J. Am. Chem. Soc. 2003, 125, 286-295
DOI: 10.1021/ja021010y |

(4-MeO,3-OMe)-tritylium ion | | E Parameter: -1.62
|  | J. Am. Chem. Soc. 2003, 125, 286-295
DOI: 10.1021/ja021010y |

(4-OMe,3-Me)-tritylium ion | | E Parameter: -1.84
|  | J. Am. Chem. Soc. 2003, 125, 286-295
DOI: 10.1021/ja021010y |

(4-MeO,4-Me)-tritylium ion | | E Parameter: -2.13
|  | J. Am. Chem. Soc. 2003, 125, 286-295
DOI: 10.1021/ja021010y |

(4-MeO)2-tritylium ion | | E Parameter: -3.04
|  | J. Am. Chem. Soc. 2003, 125, 286-295
DOI: 10.1021/ja021010y |

(4-MeO)3-tritylium ion | | E Parameter: -4.35
|  | J. Am. Chem. Soc. 2003, 125, 286-295
DOI: 10.1021/ja021010y |

(4-NMe2)-tritylium ion | | E Parameter: -7.93
|  | J. Am. Chem. Soc. 2003, 125, 286-295
DOI: 10.1021/ja021010y |

(4-NMe2)2-tritylium ion (= Malachite green) | | E Parameter: -10.29
|  | J. Am. Chem. Soc. 2003, 125, 286-295
DOI: 10.1021/ja021010y |

(4-NMe2)3-tritylium ion (= Crystal violet) | | E Parameter: -11.26
|  | J. Am. Chem. Soc. 2003, 125, 286-295
DOI: 10.1021/ja021010y |

(E)-3-(4-nitro-phenyl)-1-phenyl-prop-2-en-1-one (in DMSO) | DMSO | E Parameter: -17.33
|    | J. Am. Chem. Soc. 2011, 133, 8240-8251
DOI: 10.1021/ja200820m |

(E)-3-(4-cyano-phenyl)-1-phenyl-prop-2-en-1-one (in DMSO) | DMSO | E Parameter: -17.64
|    | J. Am. Chem. Soc. 2011, 133, 8240-8251
DOI: 10.1021/ja200820m |

(E)-1,3-diphenylprop-2-en-1-one (in DMSO) | DMSO | E Parameter: -19.39
|    | J. Am. Chem. Soc. 2017, 139, 13318-13329
DOI: 10.1021/jacs.7b05106 |

(E)-4-(4-nitrophenyl)but-3-en-2-one (in DSMO) | DMSO | E Parameter: -19.36
|  | J. Am. Chem. Soc. 2011, 133, 8240-8251
DOI: 10.1021/ja200820m |

(E)-4-methyl-1-(4-nitrophenyl)pent-1-en-3-one (in DMSO) | DMSO | E Parameter: -19.17
|  | J. Am. Chem. Soc. 2011, 133, 8240-8251
DOI: 10.1021/ja200820m |

(E)-4,4-dimethyl-1-(4-nitrophenyl)pent-1-en-3-one | DMSO | E Parameter: -19.15
|  | J. Am. Chem. Soc. 2011, 133, 8240-8251
DOI: 10.1021/ja200820m |

3-chloro-benzaldehyde (in DMSO) | DMSO | |  | J. Am. Chem. Soc. 2011, 133, 8240-8251
DOI: 10.1021/ja200820m |

3-fluoro-benzaldehyde (in DMSO) | DMSO | |  | J. Am. Chem. Soc. 2011, 133, 8240-8251
DOI: 10.1021/ja200820m |

3-methoxy-benzaldehyde (in DMSO) | DMSO | |  | J. Am. Chem. Soc. 2011, 133, 8240-8251
DOI: 10.1021/ja200820m |

4-fluoro-benzaldehyde (in DMSO) | DMSO | |  | J. Am. Chem. Soc. 2011, 133, 8240-8251
DOI: 10.1021/ja200820m |

benzaldehyde (in DMSO) | DMSO | E Parameter: -12.90
|  | J. Am. Chem. Soc. 2018, 140, 5500-5515
DOI: 10.1021/jacs.8b01657 |

hexanal (in DMSO) | DMSO | |  | J. Am. Chem. Soc. 2011, 133, 8240-8251
DOI: 10.1021/ja200820m |

butanal (in DMSO) | DMSO | |  | J. Am. Chem. Soc. 2011, 133, 8240-8251
DOI: 10.1021/ja200820m |

cinnamaldehyde (in DMSO) | DMSO | |  | J. Am. Chem. Soc. 2011, 133, 8240-8251
DOI: 10.1021/ja200820m |

p-nitro-cinnamaldehyde (in DMSO) | DMSO | |  | J. Am. Chem. Soc. 2011, 133, 8240-8251
DOI: 10.1021/ja200820m |

(E)-N-benzylidene-4-methylbenzenesulfonamide (in DMSO) | DMSO | E Parameter: -11.50
|    | J. Am. Chem. Soc. 2011, 133, 8240-8251
DOI: 10.1021/ja200820m |

(E)-N-(4-methoxybenzylidene)-4-methylbenzenesulfonamide (in DMSO) | DMSO | E Parameter: -13.05
|    | J. Am. Chem. Soc. 2011, 133, 8240-8251
DOI: 10.1021/ja200820m |

(E)-N-(4-(dimethylamino)benzylidene)-4-methylbenzenesulfonamide (in DMSO) | DMSO | E Parameter: -15.09
|    | J. Am. Chem. Soc. 2011, 133, 8240-8251
DOI: 10.1021/ja200820m |

(E)-tert-butyl benzylidenecarbamate (in DMSO) | DMSO | E Parameter: -14.22
|    | J. Am. Chem. Soc. 2011, 133, 8240-8251
DOI: 10.1021/ja200820m |

(E)-N-benzylidene-P,P-diphenylphosphinic amide | DMSO | E Parameter: -15.89
|    | J. Am. Chem. Soc. 2011, 133, 8240-8251
DOI: 10.1021/ja200820m |

(fur)(ani)CH+ | 0 | E Parameter: -0.81
|      | J. Am. Chem. Soc. 2012, 134, 13902-13911
DOI: 10.1021/ja306522b |

(mfp)PhCH+ | 0 | E Parameter: 6.23
|      | J. Am. Chem. Soc. 2012, 134, 13902-13911
DOI: 10.1021/ja306522b |

(tfm)PhCH+ | 0 | E Parameter: 6.70
|      | J. Am. Chem. Soc. 2012, 134, 13902-13911
DOI: 10.1021/ja306522b |

(dfp)PhCH+ | 0 | E Parameter: 6.74
|      | J. Am. Chem. Soc. 2012, 134, 13902-13911
DOI: 10.1021/ja306522b |

(mfp)2CH+ | 0 | E Parameter: 6.87
|      | J. Am. Chem. Soc. 2012, 134, 13902-13911
DOI: 10.1021/ja306522b |

(dfp)(mfp)CH+ | 0 | E Parameter: 7.52
|      | J. Am. Chem. Soc. 2012, 134, 13902-13911
DOI: 10.1021/ja306522b |

(tfm)2CH+ | 0 | E Parameter: 7.96
|   | J. Am. Chem. Soc. 2012, 134, 13902-13911
DOI: 10.1021/ja306522b |

(dfp)2CH+ | 0 | E Parameter: 8.02
|  | J. Am. Chem. Soc. 2012, 134, 13902-13911
DOI: 10.1021/ja306522b |

(Ftol)2CH+ | 0 | E Parameter: 5.24
|      | J. Am. Chem. Soc. 2012, 134, 13902-13911
DOI: 10.1021/ja306522b |

PhCH=N+Me2 (in MeCN) | MeCN | E Parameter: -9.27
|    | J. Am. Chem. Soc. 2013, 135, 6579-6587
DOI: 10.1021/ja401106x |

pTolCH=N+CH2 (in MeCN) | MeCN | E Parameter: -9.64
|    | J. Am. Chem. Soc. 2013, 135, 6579-6587
DOI: 10.1021/ja401106x |

p-AniCH=N+Me2 (in MeCN) | MeCN | E Parameter: -10.69
|    | J. Am. Chem. Soc. 2013, 135, 6579-6587
DOI: 10.1021/ja401106x |

PhCH=N+(CH2)4 (in MeCN) | MeCN | E Parameter: -9.35
|    | J. Am. Chem. Soc. 2013, 135, 6579-6587
DOI: 10.1021/ja401106x |

PhCH=N+(CH2)5 (in MeCN) | MeCN | E Parameter: -9.60
|    | J. Am. Chem. Soc. 2013, 135, 6579-6587
DOI: 10.1021/ja401106x |

p-CF3-C6H4-CH=N+Me2 (in MeCN) | MeCN | E Parameter: -8.34
|    | J. Am. Chem. Soc. 2013, 135, 6579-6587
DOI: 10.1021/ja401106x |

2,3-dichloro-5,6-dicyano-p-benzoquinone (DDQ) | MeCN | E Parameter: -3.66
|    | J. Am. Chem. Soc. 2013, 135, 12377-12387
DOI: 10.1021/ja405890d |

p-quinone (C-H) | 0 | E Parameter: -16.19
|   | J. Am. Chem. Soc. 2014, 136, 11499-11512
DOI: 10.1021/ja505613b |

2,5-dichloroquinone (C-H) | 0 | E Parameter: -12.28
|    | J. Am. Chem. Soc. 2014, 136, 11499-11512
DOI: 10.1021/ja505613b |

2,5-dichloroquinone (C-Cl) | 0 | E Parameter: -16.11
|    | J. Am. Chem. Soc. 2014, 136, 11499-11512
DOI: 10.1021/ja505613b |

p-chloranil (C=O) | 0 | E Parameter: -12.13
|    | J. Am. Chem. Soc. 2014, 136, 11499-11512
DOI: 10.1021/ja505613b |

p-chloranil (C-Cl) | 0 | E Parameter: -13.84
|    | J. Am. Chem. Soc. 2014, 136, 11499-11512
DOI: 10.1021/ja505613b |

p-fluoranil (C=O) | 0 | E Parameter: -9.37
|    | J. Am. Chem. Soc. 2014, 136, 11499-11512
DOI: 10.1021/ja505613b |

p-fluoranil (C-F) | 0 | E Parameter: -11.12
|    | J. Am. Chem. Soc. 2014, 136, 11499-11512
DOI: 10.1021/ja505613b |

o-chloranil (C=O) | 0 | E Parameter: -8.77
|    | J. Am. Chem. Soc. 2014, 136, 11499-11512
DOI: 10.1021/ja505613b |

o-chloranil (C-Cl) | 0 | E Parameter: -12.02
|    | J. Am. Chem. Soc. 2014, 136, 11499-11512
DOI: 10.1021/ja505613b |

(E)-4-phenylbut-3-en-2-one (in DMSO) | DMSO | E Parameter: -23.01
|    | J. Am. Chem. Soc. 2017, 139, 13318-13329
DOI: 10.1021/jacs.7b05106 |

cinnamonitrile (in DMSO) | DMSO | E Parameter: -24.60
|  | J. Am. Chem. Soc. 2017, 139, 13318-13329
DOI: 10.1021/jacs.7b05106 |

methyl prop-2-enoate (in DMSO) | DMSO | E Parameter: -18.84
|    | J. Am. Chem. Soc. 2017, 139, 13318-13329
DOI: 10.1021/jacs.7b05106 |

ethyl prop-2-enoate (in DMSO) | DMSO | E Parameter: -19.07
|    | J. Am. Chem. Soc. 2017, 139, 13318-13329
DOI: 10.1021/jacs.7b05106 |

tert-butyl prop-2-enoate (in DMSO) | DMSO | E Parameter: -20.22
|    | J. Am. Chem. Soc. 2017, 139, 13318-13329
DOI: 10.1021/jacs.7b05106 |

N,N-dimethylprop-2-enamide (in DMSO) | DMSO | E Parameter: -23.54
|    | J. Am. Chem. Soc. 2017, 139, 13318-13329
DOI: 10.1021/jacs.7b05106 |

ethenylsulfonylbenzene (in DMSO) | DMSO | E Parameter: -18.36
|    | J. Am. Chem. Soc. 2017, 139, 13318-13329
DOI: 10.1021/jacs.7b05106 |

acrylonitrile (in DMSO) | DMSO | E Parameter: -19.05
|    | J. Am. Chem. Soc. 2017, 139, 13318-13329
DOI: 10.1021/jacs.7b05106 |

but-3-en-2-one (in DMSO) | DMSO | E Parameter: -16.76
|    | J. Am. Chem. Soc. 2017, 139, 13318-13329
DOI: 10.1021/jacs.7b05106 |

1-phenylprop-2-en-1-one (in DMSO) | DMSO | E Parameter: -15.25
|    | J. Am. Chem. Soc. 2017, 139, 13318-13329
DOI: 10.1021/jacs.7b05106 |

ethyl 2-methylprop-2-enoate (in DMSO) | DMSO | E Parameter: -22.77
|   | J. Am. Chem. Soc. 2017, 139, 13318-13329
DOI: 10.1021/jacs.7b05106 |

ethyl (E)-but-2-enoate (in DMSO) | DMSO | E Parameter: -23.59
|   | J. Am. Chem. Soc. 2017, 139, 13318-13329
DOI: 10.1021/jacs.7b05106 |

ethyl cinnamate (in DMSO) | DMSO | E Parameter: -24.52
|   | J. Am. Chem. Soc. 2017, 139, 13318-13329
DOI: 10.1021/jacs.7b05106 |

(E)-1-methyl-4-(styrylsulfonyl)benzene (in DMSO) | DMSO | E Parameter: -24.69
|   | J. Am. Chem. Soc. 2017, 139, 13318-13329
DOI: 10.1021/jacs.7b05106 |

cyclobutanone (in DMSO) | DMSO | E Parameter: -17.50
|  | J. Am. Chem. Soc. 2018, 140, 5500-5515
DOI: 10.1021/jacs.8b01657 |

cyclopentanone (in DMSO) | DMSO | E Parameter: -21.00
|  | J. Am. Chem. Soc. 2018, 140, 5500-5515
DOI: 10.1021/jacs.8b01657 |

cyclohexanone (in DMSO) | DMSO | E Parameter: -19.90
|  | J. Am. Chem. Soc. 2018, 140, 5500-5515
DOI: 10.1021/jacs.8b01657 |

cycloheptanone (in DMSO) | DMSO | E Parameter: -22.10
|  | J. Am. Chem. Soc. 2018, 140, 5500-5515
DOI: 10.1021/jacs.8b01657 |

1-methylpiperidin-4-one (in DMSO) | DMSO | E Parameter: -18.40
|  | J. Am. Chem. Soc. 2018, 140, 5500-5515
DOI: 10.1021/jacs.8b01657 |

oxan-4-one (in DMSO) | DMSO | E Parameter: -17.90
|  | J. Am. Chem. Soc. 2018, 140, 5500-5515
DOI: 10.1021/jacs.8b01657 |

thian-4-one (in DMSO) | DMSO | E Parameter: -16.90
|  | J. Am. Chem. Soc. 2018, 140, 5500-5515
DOI: 10.1021/jacs.8b01657 |

1,4-dioxaspiro[4.5]decan-8-one (in DMSO) | DMSO | E Parameter: -18.20
|  | J. Am. Chem. Soc. 2018, 140, 5500-5515
DOI: 10.1021/jacs.8b01657 |

pentan-2-one (in DMSO) | DMSO | E Parameter: -22.30
|  | J. Am. Chem. Soc. 2018, 140, 5500-5515
DOI: 10.1021/jacs.8b01657 |

1-methylsulfanylpropan-2-one (in DMSO) | DMSO | E Parameter: -15.60
|  | J. Am. Chem. Soc. 2018, 140, 5500-5515
DOI: 10.1021/jacs.8b01657 |

N-fluoro-2,4,6-trimethylpyridinium tetrafluoroborate | 0 | E Parameter: -10.46
|    | J. Am. Chem. Soc. 2018, 140, 11474-11486
DOI: 10.1021/jacs.8b07147 |

N-fluoro-2,6-dichloropyridinium tetrafluoroborate | 0 | E Parameter: -5.29
|    | J. Am. Chem. Soc. 2018, 140, 11474-11486
DOI: 10.1021/jacs.8b07147 |

Selectfluor | 0 | E Parameter: -5.20
|    | J. Am. Chem. Soc. 2018, 140, 11474-11486
DOI: 10.1021/jacs.8b07147 |

NFSI | MeCN | E Parameter: -8.44
|    | J. Am. Chem. Soc. 2018, 140, 11474-11486
DOI: 10.1021/jacs.8b07147 |

N-fluoropyridinium tetrafluoroborate | 0 | E Parameter: -9.89
|    | J. Am. Chem. Soc. 2018, 140, 11474-11486
DOI: 10.1021/jacs.8b07147 |

phenyl isocyanate | MeCN | E Parameter: -15.38
|    | J. Am. Chem. Soc. 2020, 142, 8383-8402
DOI: 10.1021/jacs.0c01960 |

p-tosyl isocyanate | MeCN | E Parameter: -7.69
|    | J. Am. Chem. Soc. 2020, 142, 8383-8402
DOI: 10.1021/jacs.0c01960 |

phenyl isothiocyanate | DMSO | E Parameter: -18.15
|    | J. Am. Chem. Soc. 2020, 142, 8383-8402
DOI: 10.1021/jacs.0c01960 |

p-nitrophenyl isothiocyanate | DMSO | E Parameter: -15.89
|    | J. Am. Chem. Soc. 2020, 142, 8383-8402
DOI: 10.1021/jacs.0c01960 |

diphenylcarbodiimide | DMSO | E Parameter: -20.14
|    | J. Am. Chem. Soc. 2020, 142, 8383-8402
DOI: 10.1021/jacs.0c01960 |

carbon disulfide | DMSO | E Parameter: -17.70
|    | J. Am. Chem. Soc. 2020, 142, 8383-8402
DOI: 10.1021/jacs.0c01960 |

benzylidenemalononitrile | 0 | E Parameter: -9.42
|    | J. Org. Chem. 2003, 68, 6880-6886
DOI: 10.1021/jo0344182 |

p-(methoxy)benzylidenemalononitrile | 0 | E Parameter: -10.80
|    | J. Org. Chem. 2003, 68, 6880-6886
DOI: 10.1021/jo0344182 |

p-(dimethylamino)benzylidenemalononitrile | 0 | E Parameter: -13.30
|    | J. Org. Chem. 2003, 68, 6880-6886
DOI: 10.1021/jo0344182 |

4,6-dinitrobenzofuroxan | 0 | E Parameter: -5.06
|    | J. Org. Chem. 2006, 71, 9088-9095
DOI: 10.1021/jo0614339 |

1,3,5-trinitrobenzene | 0 | E Parameter: -13.19
|    | J. Org. Chem. 2005, 70, 6242-6253
DOI: 10.1021/jo0505526 |

6-nitro-tetrazolo[1,5a]pyridine | 0 | E Parameter: -9.05
|    | J. Org. Chem. 2005, 70, 6242-6253
DOI: 10.1021/jo0505526 |

4-nitro-6-(trifluoromethylsulfonyl)benzofuroxan | 0 | E Parameter: -4.91
|    | J. Org. Chem. 2005, 70, 6242-6253
DOI: 10.1021/jo0505526 |

4,6-dinitrotetrazolopyridine | 0 | E Parameter: -4.67
|    | J. Org. Chem. 2005, 70, 6242-6253
DOI: 10.1021/jo0505526 |

4,6-dinitrobenzofurazan | 0 | E Parameter: -5.46
|    | J. Org. Chem. 2005, 70, 6242-6253
DOI: 10.1021/jo0505526 |

2,4-dinitrothiophene | 0 | E Parameter: -12.33
|    | J. Org. Chem. 2005, 70, 6242-6253
DOI: 10.1021/jo0505526 |

6-cyano-4-nitrobenzofuroxan | 0 | E Parameter: -7.01
|    | J. Org. Chem. 2005, 70, 6242-6253
DOI: 10.1021/jo0505526 |

4-cyano-6-nitro-benzofuroxane | 0 | E Parameter: -6.41
|    | J. Org. Chem. 2005, 70, 6242-6253
DOI: 10.1021/jo0505526 |

4-aza-6-nitrobenzofuroxan | 0 | E Parameter: -5.86
|    | J. Org. Chem. 2005, 70, 6242-6253
DOI: 10.1021/jo0505526 |

aniBBS | 0 | E Parameter: -10.37
|    | J. Org. Chem. 2007, 72, 9170-9180
DOI: 10.1021/jo071273g |

dmaBBS | 0 | E Parameter: -12.76
|    | J. Org. Chem. 2007, 72, 9170-9180
DOI: 10.1021/jo071273g |

julBBS | 0 | E Parameter: -13.84
|    | J. Org. Chem. 2007, 72, 9170-9180
DOI: 10.1021/jo071273g |

dma(S)BBS | 0 | E Parameter: -10.73
|    | J. Org. Chem. 2007, 72, 9170-9180
DOI: 10.1021/jo071273g |

jul(S)BBS | 0 | E Parameter: -11.89
|    | J. Org. Chem. 2007, 72, 9170-9180
DOI: 10.1021/jo071273g |

benzylidene-Meldrum's acid | 0 | E Parameter: -9.15
|    | J. Org. Chem. 2008, 73, 2738-2745
DOI: 10.1021/jo702590s |

p-(methoxy)benzylidene Meldrum's acid | 0 | E Parameter: -10.28
|    | J. Org. Chem. 2008, 73, 2738-2745
DOI: 10.1021/jo702590s |

p-(dimethylamino)benzylidene Meldrum's acid | 0 | E Parameter: -12.76
|    | J. Org. Chem. 2008, 73, 2738-2745
DOI: 10.1021/jo702590s |

jul Meldrum's acid | 0 | E Parameter: -13.97
|    | J. Org. Chem. 2008, 73, 2738-2745
DOI: 10.1021/jo702590s |

diarylallylium ion (3,3-F2)2 | | E Parameter: 6.11
|    | J. Org. Chem. 2011, 76, 9391-9408
DOI: 10.1021/jo201668w |

diarylallylium ion (3-F)2 | | E Parameter: 4.15
|   | J. Org. Chem. 2011, 76, 9391-9408
DOI: 10.1021/jo201668w |

diarylallylium ion (4-Br)2 | | E Parameter: 2.85
|    | J. Org. Chem. 2011, 76, 9391-9408
DOI: 10.1021/jo201668w |

diarylallylium ion (4-Cl)2 | | E Parameter: 2.69
|  | J. Org. Chem. 2011, 76, 9391-9408
DOI: 10.1021/jo201668w |

diphenylallylium ion | | E Parameter: 2.70
|    | J. Org. Chem. 2011, 76, 9391-9408
DOI: 10.1021/jo201668w |

di-p-tolyl-allylium ion | | E Parameter: 1.23
|    | J. Org. Chem. 2011, 76, 9391-9408
DOI: 10.1021/jo201668w |

di-p-anisyl-allylium ion | | E Parameter: -1.45
|    | J. Org. Chem. 2011, 76, 9391-9408
DOI: 10.1021/jo201668w |

diarylallylium ion (4-NMe2)2 | | E Parameter: -7.50
|    | J. Org. Chem. 2011, 76, 9391-9408
DOI: 10.1021/jo201668w |

bis(julidin-9-yl)allylium ion | | E Parameter: -9.78
|    | J. Org. Chem. 2011, 76, 9391-9408
DOI: 10.1021/jo201668w |

4-methoxy-<i>trans-beta</i>-nitrostyrene | | E Parameter: -14.70
|    | J. Org. Chem. 2011, 76, 9370-9378
DOI: 10.1021/jo201678u |

4-methyl-<i>trans-beta</i>-nitrostyrene | | E Parameter: -14.23
|    | J. Org. Chem. 2011, 76, 9370-9378
DOI: 10.1021/jo201678u |

<i>trans-beta</i>-nitrostyrene | | E Parameter: -13.85
|    | J. Org. Chem. 2011, 76, 9370-9378
DOI: 10.1021/jo201678u |

4-bromo-<i>trans-beta</i>-nitrostyrene | | E Parameter: -13.37
|    | J. Org. Chem. 2011, 76, 9370-9378
DOI: 10.1021/jo201678u |

4-cyano-<i>trans-beta</i>-nitrostyrene | | E Parameter: -12.61
|    | J. Org. Chem. 2011, 76, 9370-9378
DOI: 10.1021/jo201678u |

4-nitro-<i>trans-beta</i>-nitrostyrene | | E Parameter: -12.37
|    | J. Org. Chem. 2011, 76, 9370-9378
DOI: 10.1021/jo201678u |

3-benzylidene-1-methyl-3H-indol-1-ium ion | 0 | E Parameter: -1.80
|    | J. Org. Chem. 2015, 80, 8643-8656
DOI: 10.1021/acs.joc.5b01298 |

1-methyl-3-(4-methylbenzylidene)-3H-indol-1-ium ion | 0 | E Parameter: -2.19
|    | J. Org. Chem. 2015, 80, 8643-8656
DOI: 10.1021/acs.joc.5b01298 |

3-(4-methoxybenzylidene)-1-methyl-3H-indol-1-ium iom | 0 | E Parameter: -3.02
|    | J. Org. Chem. 2015, 80, 8643-8656
DOI: 10.1021/acs.joc.5b01298 |

3-(4-(dimethylamino)benzylidene)-1-methyl-3H-indol-1-ium ion | 0 | E Parameter: -6.26
|    | J. Org. Chem. 2015, 80, 8643-8656
DOI: 10.1021/acs.joc.5b01298 |

1-methyl-3-((julolidin-9-yl)methylene)-3H-indol-1-ium ion | 0 | E Parameter: -7.79
|    | J. Org. Chem. 2015, 80, 8643-8656
DOI: 10.1021/acs.joc.5b01298 |

(E)-2-methyl-3-(4-methylbenzylidene)-3H-indol-1-ium ion | 0 | E Parameter: -5.15
|    | J. Org. Chem. 2015, 80, 8643-8656
DOI: 10.1021/acs.joc.5b01298 |

(E)-1,2-dimethyl-3-(4-methylbenzylidene)-3H-indol-1-ium ion | 0 | E Parameter: -5.05
|    | J. Org. Chem. 2015, 80, 8643-8656
DOI: 10.1021/acs.joc.5b01298 |

(E)-3-benzylidene-1,2-dimethyl-3H-indol-1-ium ion | 0 | E Parameter: -4.96
|   | J. Org. Chem. 2015, 80, 8643-8656
DOI: 10.1021/acs.joc.5b01298 |

bis(1-methylindol-3-yl)methylium ion | 0 | E Parameter: -5.99
|    | J. Org. Chem. 2015, 80, 8643-8656
DOI: 10.1021/acs.joc.5b01298 |

bis(5-methoxy-1-methylindol-3-yl)methylium ion | 0 | E Parameter: -6.90
|    | J. Org. Chem. 2015, 80, 8643-8656
DOI: 10.1021/acs.joc.5b01298 |

bis(1,2-dimethylindol-3-yl)methylium ion | 0 | E Parameter: -10.23
|    | J. Org. Chem. 2015, 80, 8643-8656
DOI: 10.1021/acs.joc.5b01298 |

5-methoxyfuroxano[3,4-d]pyrimidine | 0 | E Parameter: -8.37
|    | J. Phys. Org. Chem. 2003, 16, 431-437
DOI: 10.1002/poc.606 |
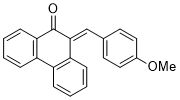
(E)-10-(4-methoxybenzylidene)phenanthren-9(10H)-one | 0 | E Parameter: -15.93
|    | J. Phys. Org. Chem. 2025, , accepted
DOI: 10.1002/poc.70061 |
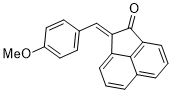
(E)-2-(4-methoxybenzylidene)acenaphthylen-1(2H)-one | 0 | E Parameter: -18.72
|    | J. Phys. Org. Chem. 2025, , accepted
DOI: 10.1002/poc.70061 |

dibenzo[a,d]tropylium ion | dichloromethane | E Parameter: -0.63
|  | Liebigs Ann. 1995, , 2005-2009
DOI: 10.1002/jlac.1995199511281 |

(2,4,6-trimethylphenyl)ethylium ion | 0 | E Parameter: 6.04
|  | Macromolecules 2005, 38, 33-40
DOI: 10.1021/ma048389o |

1,3-bis(2,4,6-trimethylphenyl)but-1-ylium ion | 0 | E Parameter: 6.16
|  | Macromolecules 2005, 38, 33-40
DOI: 10.1021/ma048389o |

cumyl cation | | E Parameter: 5.74
|    | Macromolecules 2010, 43, 1719-1723
DOI: 10.1021/ma9024569 |

2-(p-(dimethylamino)benzylidene)-indan-1,3-dione | 0 | E Parameter: -13.56
|    | Org. Biomol. Chem. 2007, 5, 3020-3026
DOI: 10.1039/b708025e |

2-(p-methoxybenzylidene)-indan-1,3-dione | 0 | E Parameter: -11.32
|    | Org. Biomol. Chem. 2007, 5, 3020-3026
DOI: 10.1039/b708025e |

2-benzylidene-indan-1,3-dione | 0 | E Parameter: -10.11
|    | Org. Biomol. Chem. 2007, 5, 3020-3026
DOI: 10.1039/b708025e |

jul-indan-1,3-dione | 0 | E Parameter: -14.68
|    | Org. Biomol. Chem. 2007, 5, 3020-3026
DOI: 10.1039/b708025e |
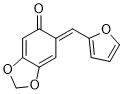
(E)-6-(furan-2-ylmethylene)benzo[d][1,3]dioxol-5(6H)-one | 0 | E Parameter: -15.73
|    | Org. Biomol. Chem. 2025, 23, 827-834
DOI: 10.1039/D4OB01855A |
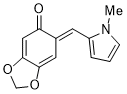
(E)-6-((1-methyl-1H-pyrrol-2-yl)methylene)benzo[d][1,3]dioxol-5(6H)-one | 0 | E Parameter: -17.03
|    | Org. Biomol. Chem. 2025, 23, 827-834
DOI: 10.1039/D4OB01855A |
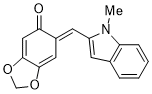
(E)-6-((1-methyl-1H-indol-2-yl)methylene)benzo[d][1,3]dioxol-5(6H)-one | 0 | E Parameter: -15.55
|    | Org. Biomol. Chem. 2025, 23, 827-834
DOI: 10.1039/D4OB01855A |
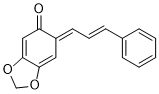
(E)-6-((E)-3-phenylallylidene)benzo[d][1,3]dioxol-5(6H)-one | 0 | E Parameter: -16.00
|    | Org. Biomol. Chem. 2025, 23, 827-834
DOI: 10.1039/D4OB01855A |

2,3,4,5,6,6-hexachlorocyclohexa-2,4-dien-1-one | MeCN | E Parameter: -6.75
|    | Org. Lett. 2010, 12, 2238-2241
DOI: 10.1021/ol100592j |

5,7,7-trichloro-8(7H)-quinolinone | MeCN | E Parameter: -10.48
|    | Org. Lett. 2010, 12, 2238-2241
DOI: 10.1021/ol100592j |

2,2,4-trichloro-1(2H)-naphthalenone | MeCN | E Parameter: -11.24
|    | Org. Lett. 2010, 12, 2238-2241
DOI: 10.1021/ol100592j |

(E)-2,6-di-tert-butyl-4-(3-phenylallylidene)cyclohexa-2,5-dien-1-one | DMSO | E Parameter: -17.00
|    | Org. Lett. 2020, 22, 2182-2186
DOI: 10.1021/acs.orglett.0c00338 |

E)-2,6-di-tert-butyl-4-(3-(4-chlorophenyl)allylidene)cyclohexa-2,5-dien-1-one | DMSO | E Parameter: -16.84
|    | Org. Lett. 2020, 22, 2182-2186
DOI: 10.1021/acs.orglett.0c00338 |

(E)-2,6-di-tert-butyl-4-(3-(4-nitrophenyl)allylidene)cyclohexa-2,5-dien-1-one | DMSO | E Parameter: -16.25
|    | Org. Lett. 2020, 22, 2182-2186
DOI: 10.1021/acs.orglett.0c00338 |

(E)-2,6-di-tert-butyl-4-(3-(4-methoxyphenyl)allylidene)cyclohexa-2,5-dien-1-one | DMSO | E Parameter: -17.42
|    | Org. Lett. 2020, 22, 2182-2186
DOI: 10.1021/acs.orglett.0c00338 |

[(1,3-Diarylallyl)Pd(PPh3)2]+ (Aryl = Ph) | | E Parameter: -14.14
|    | Organometallics 2012, 31, 2416-2424
DOI: 10.1021/om3000357 |

[(1,3-Diarylallyl)Pd(PPh3)2]+ (Aryl = 3,5-difluorophenyl) | | E Parameter: -14.21
|    | Organometallics 2012, 31, 2416-2424
DOI: 10.1021/om3000357 |

[(1,3-Diarylallyl)Pd(PPh3)2]+ (Aryl = 4-(dimethylamino)phenyl) | | E Parameter: -14.46
|    | Organometallics 2012, 31, 2416-2424
DOI: 10.1021/om3000357 |

Me2C=C=N(+)Me2 | 0 | E Parameter: -2.80
|  | Synthesis 2025, 57, 3251-3262
DOI: 10.1055/a-2649-1999 |

Ph2C=C=N(+)Me2 | 0 | E Parameter: -1.90
|  | Synthesis 2025, 57, 3251-3262
DOI: 10.1055/a-2649-1999 |

2-cinnamoyl-3-methyl-1-(perfluorophenyl)-1H-imidazol-3-ium | DMSO | E Parameter: -10.09
|    | Top. Catal. 2018, 61, 585-590
DOI: 10.1007/s11244-018-0914-5 |

1-(tert-butyl)-2-cinnamoyl-3-methyl-1H-imidazol-3-ium | DMSO | E Parameter: -11.80
|    | Top. Catal. 2018, 61, 585-590
DOI: 10.1007/s11244-018-0914-5 |

2-cinnamoyl-1-mesityl-3-methyl-1H-imidazol-3-ium | DMSO | E Parameter: -11.48
|    | Top. Catal. 2018, 61, 585-590
DOI: 10.1007/s11244-018-0914-5 |

2-cinnamoyl-1-(4-methoxyphenyl)-3-methyl-1H-imidazol-3-ium | DMSO | E Parameter: -11.79
|    | Top. Catal. 2018, 61, 585-590
DOI: 10.1007/s11244-018-0914-5 |

2-cinnamoyl-1-(2,6-dimethoxyphenyl)-3-methyl-1H-imidazol-3-ium | DMSO | E Parameter: -12.02
|    | Top. Catal. 2018, 61, 585-590
DOI: 10.1007/s11244-018-0914-5 |