N-Nucleophiles
For reactivities of structurally related compounds go to: Nucleophiles
Found 342 Molecules.
| Molecule | Solvent | Reactivity Parameters | Classification | Reference (sort by title or year) |
|---|---|---|---|---|
 thiocyanate (in MeCN) | MeCN | N Parameter: 12.13 sN Parameter: 0.60 | J. Am. Chem. Soc. 2003, 125, 14126-14132 DOI: 10.1021/ja037317u | |
 4-chloropyridine (in CH2Cl2) | dichloromethane | N Parameter: 11.70 sN Parameter: 0.67 | Chem. Eur. J. 2007, 13, 336-345 DOI: 10.1002/chem.200600941 | |
 4-chloropyridine (in H2O) | water | N Parameter: 10.50 sN Parameter: 0.73 | Chem. Eur. J. 2007, 13, 336-345 DOI: 10.1002/chem.200600941 | |
 pyridine (in CH2Cl2) | dichloromethane | N Parameter: 12.90 sN Parameter: 0.67 | Chem. Eur. J. 2007, 13, 336-345 DOI: 10.1002/chem.200600941 | |
 4-methylpyridine (in CH2Cl2) | dichloromethane | N Parameter: 13.70 sN Parameter: 0.67 | Chem. Eur. J. 2007, 13, 336-345 DOI: 10.1002/chem.200600941 | |
 4-methoxypyridine (in CH2Cl2) | dichloromethane | N Parameter: 13.70 sN Parameter: 0.67 | Chem. Eur. J. 2007, 13, 336-345 DOI: 10.1002/chem.200600941 | |
 4-aminopyridine (in CH2Cl2) | dichloromethane | N Parameter: 15.20 sN Parameter: 0.67 | Chem. Eur. J. 2007, 13, 336-345 DOI: 10.1002/chem.200600941 | |
 4-(dimethylamino)pyridine (in 91M9AN) | MeOH-MeCN mix | N Parameter: 13.20 sN Parameter: 0.67 | Chem. Eur. J. 2007, 13, 336-345 DOI: 10.1002/chem.200600941 | |
 4-(dimethylamino)pyridine (in DMSO) | DMSO | N Parameter: 14.80 sN Parameter: 0.67 | Chem. Eur. J. 2007, 13, 336-345 DOI: 10.1002/chem.200600941 | |
 4-(dimethylamino)pyridine (in DMF) | DMF | N Parameter: 14.90 sN Parameter: 0.67 | Chem. Eur. J. 2007, 13, 336-345 DOI: 10.1002/chem.200600941 | |
 4-pyrrolidinopyridine (in CH2Cl2) | dichloromethane | N Parameter: 15.90 sN Parameter: 0.67 | Chem. Eur. J. 2007, 13, 336-345 DOI: 10.1002/chem.200600941 | |
 5-methoxy-benzimidazole (in DMSO) | DMSO | N Parameter: 11.00 sN Parameter: 0.71 | Org. Biomol. Chem. 2010, 8, 1929-1935 DOI: 10.1039/c000965b | |
 benzotriazole (in MeCN) | MeCN | N Parameter: 7.69 sN Parameter: 0.76 | Org. Biomol. Chem. 2010, 8, 1929-1935 DOI: 10.1039/c000965b | |
 1-methyl-benzotriazole (in MeCN) | MeCN | N Parameter: 7.77 sN Parameter: 0.76 | Org. Biomol. Chem. 2010, 8, 1929-1935 DOI: 10.1039/c000965b | |
 triethylamine (in MeCN) | MeCN | N Parameter: 17.10 sN Parameter: 0.52 | J. Phys. Org. Chem. 2010, 23, 1029-1035 DOI: 10.1002/poc.1707 | |
 triethylamine (in CH2Cl2) | dichloromethane | N Parameter: 17.30 sN Parameter: 0.52 | J. Phys. Org. Chem. 2010, 23, 1029-1035 DOI: 10.1002/poc.1707 | |
 N-methyl-pyrrolidine (in CH2Cl2) | dichloromethane | N Parameter: 20.60 sN Parameter: 0.52 | J. Phys. Org. Chem. 2010, 23, 1029-1035 DOI: 10.1002/poc.1707 | |
 N-methyl-piperidine (in CH2Cl2) | dichloromethane | N Parameter: 18.90 sN Parameter: 0.52 | J. Phys. Org. Chem. 2010, 23, 1029-1035 DOI: 10.1002/poc.1707 | |
 N-methyl-morpholine (in MeCN) | MeCN | N Parameter: 16.80 sN Parameter: 0.52 | J. Phys. Org. Chem. 2010, 23, 1029-1035 DOI: 10.1002/poc.1707 | |
 N-methyl-morpholine (in CH2Cl2) | dichloromethane | N Parameter: 16.50 sN Parameter: 0.52 | J. Phys. Org. Chem. 2010, 23, 1029-1035 DOI: 10.1002/poc.1707 | |
 1-phenyl-N-(phenylmethyl)methanimine (in CH2Cl2) | dichloromethane | N Parameter: 7.90 sN Parameter: 0.76 | Z. Naturforsch. B 2013, 68b, 693-699 DOI: 10.5560/ZNB.2013-3085 | |
 semicarbazide (in water) | water | N Parameter: 11.05 sN Parameter: 0.52 | J. Am. Chem. Soc. 2003, 125, 286-295 DOI: 10.1021/ja021010y | |
 hydroxylamine (in water) | water | N Parameter: 11.41 sN Parameter: 0.55 | J. Am. Chem. Soc. 2003, 125, 286-295 DOI: 10.1021/ja021010y | |
 2,2,2-trifluoroethylamine (in DMSO) | DMSO | N Parameter: 12.15 sN Parameter: 0.65 | J. Am. Chem. Soc. 2003, 125, 286-295 DOI: 10.1021/ja021010y | |
 n-propylamine (in water) | water | N Parameter: 13.33 sN Parameter: 0.56 | J. Am. Chem. Soc. 2003, 125, 286-295 DOI: 10.1021/ja021010y | |
 ethyl glycinate (in DMSO) | DMSO | N Parameter: 14.30 sN Parameter: 0.67 | J. Am. Chem. Soc. 2003, 125, 286-295 DOI: 10.1021/ja021010y | |
 n-propylamine (in DMSO) | DMSO | N Parameter: 15.70 sN Parameter: 0.64 | J. Am. Chem. Soc. 2003, 125, 286-295 DOI: 10.1021/ja021010y | |
 morpholine (in DMSO) | DMSO | N Parameter: 16.96 sN Parameter: 0.67 | J. Am. Chem. Soc. 2003, 125, 286-295 DOI: 10.1021/ja021010y | |
 piperidine (in DMSO) | DMSO | N Parameter: 17.19 sN Parameter: 0.71 | J. Am. Chem. Soc. 2003, 125, 286-295 DOI: 10.1021/ja021010y | |
 nitrite ion (in MeCN) | MeCN | N Parameter: 17.20 sN Parameter: 0.72 | Angew. Chem. Int. Ed. 2005, 44, 4623-4626 DOI: 10.1002/anie.200501274 | |
 azide ion (in 91M9AN) | MeOH-MeCN mix | N Parameter: 14.54 sN Parameter: 0.82 | J. Phys. Org. Chem. 2006, 19, 706-713 DOI: 10.1002/poc.1063 | |
 azide ion (in 91E9AN) | EtOH-MeCN mix | N Parameter: 16.30 sN Parameter: 0.73 | J. Phys. Org. Chem. 2006, 19, 706-713 DOI: 10.1002/poc.1063 | |
 azide ion (in 91nPr9AN) | nPrOH-MeCN mix | N Parameter: 16.70 sN Parameter: 0.73 | J. Phys. Org. Chem. 2006, 19, 706-713 DOI: 10.1002/poc.1063 | |
 azide ion (in 91iPr9AN) | iPrOH-MeCN mix | N Parameter: 17.07 sN Parameter: 0.71 | J. Phys. Org. Chem. 2006, 19, 706-713 DOI: 10.1002/poc.1063 | |
 azide ion (in DMSO) | DMSO | N Parameter: 20.50 sN Parameter: 0.59 | J. Phys. Org. Chem. 2006, 19, 706-713 DOI: 10.1002/poc.1063 | |
 azide ion (in 45M55AN) | MeOH-MeCN mix | N Parameter: 15.01 sN Parameter: 0.80 | J. Phys. Org. Chem. 2006, 19, 706-713 DOI: 10.1002/poc.1063 | |
 pyrrolidine (in 91M9AN) | MeOH-MeCN mix | N Parameter: 15.97 sN Parameter: 0.63 | Angew. Chem. Int. Ed. 2006, 45, 3869-3874 DOI: 10.1002/anie.200600542 | |
 piperidine (in 91M9AN) | MeOH-MeCN mix | N Parameter: 15.63 sN Parameter: 0.64 | Angew. Chem. Int. Ed. 2006, 45, 3869-3874 DOI: 10.1002/anie.200600542 | |
 morpholine (in 91M9AN) | MeOH-MeCN mix | N Parameter: 15.40 sN Parameter: 0.64 | Angew. Chem. Int. Ed. 2006, 45, 3869-3874 DOI: 10.1002/anie.200600542 | |
 diethanolamine (in 91M9AN) | MeOH-MeCN mix | N Parameter: 13.71 sN Parameter: 0.67 | Angew. Chem. Int. Ed. 2006, 45, 3869-3874 DOI: 10.1002/anie.200600542 | |
 hydrazine (in 91M9AN) | MeOH-MeCN mix | N Parameter: 13.47 sN Parameter: 0.70 | Angew. Chem. Int. Ed. 2006, 45, 3869-3874 DOI: 10.1002/anie.200600542 | |
 2,2,2-trifluoroethylamine (in 91M9AN) | MeOH-MeCN mix | N Parameter: 10.20 sN Parameter: 0.92 | Angew. Chem. Int. Ed. 2006, 45, 3869-3874 DOI: 10.1002/anie.200600542 | |
 imidazole (in 91M9AN) | MeOH-MeCN mix | N Parameter: 10.41 sN Parameter: 0.70 | Angew. Chem. Int. Ed. 2006, 45, 3869-3874 DOI: 10.1002/anie.200600542 | |
 hydroxylamine (in 91M9AN) | MeOH-MeCN mix | N Parameter: 12.23 sN Parameter: 0.66 | Angew. Chem. Int. Ed. 2006, 45, 3869-3874 DOI: 10.1002/anie.200600542 | |
 ethanolamine (in 91M9AN) | MeOH-MeCN mix | N Parameter: 13.23 sN Parameter: 0.65 | Angew. Chem. Int. Ed. 2006, 45, 3869-3874 DOI: 10.1002/anie.200600542 | |
 benzylamine (in 91M9AN) | MeOH-MeCN mix | N Parameter: 13.46 sN Parameter: 0.62 | Angew. Chem. Int. Ed. 2006, 45, 3869-3874 DOI: 10.1002/anie.200600542 | |
 n-propylamine (in 91M9AN) | MeOH-MeCN mix | N Parameter: 13.41 sN Parameter: 0.66 | Angew. Chem. Int. Ed. 2006, 45, 3869-3874 DOI: 10.1002/anie.200600542 | |
 pyridine (in H2O) | water | N Parameter: 11.05 sN Parameter: 0.73 | Chem. Eur. J. 2007, 13, 336-345 DOI: 10.1002/chem.200600941 | |
 4-methylpyridine (in H2O) | water | N Parameter: 11.10 sN Parameter: 0.75 | Chem. Eur. J. 2007, 13, 336-345 DOI: 10.1002/chem.200600941 | |
 4-methoxypyridine (in H2O) | water | N Parameter: 11.44 sN Parameter: 0.68 | Chem. Eur. J. 2007, 13, 336-345 DOI: 10.1002/chem.200600941 | |
 4-aminopyridine (in H2O) | water | N Parameter: 12.19 sN Parameter: 0.66 | Chem. Eur. J. 2007, 13, 336-345 DOI: 10.1002/chem.200600941 | |
 4-(dimethylamino)pyridine (in CH2Cl2) | dichloromethane | N Parameter: 15.80 sN Parameter: 0.66 | Chem. Eur. J. 2007, 13, 336-345 DOI: 10.1002/chem.200600941 | |
 4-(dimethylamino)pyridine (in MeCN) | MeCN | N Parameter: 15.51 sN Parameter: 0.62 | J. Phys. Chem. A 2012, 116, 8494-8499 DOI: 10.1021/jp3049247 | |
 4-(dimethylamino)pyridine (in H2O) | water | N Parameter: 13.19 sN Parameter: 0.56 | Chem. Eur. J. 2007, 13, 336-345 DOI: 10.1002/chem.200600941 | |
 4-pyrrolidinopyridine (in H2O) | water | N Parameter: 12.39 sN Parameter: 0.66 | Chem. Eur. J. 2007, 13, 336-345 DOI: 10.1002/chem.200600941 | |
 ammonia (in water) | water | N Parameter: 9.48 sN Parameter: 0.59 | J. Org. Chem. 2007, 72, 3679-3688 DOI: 10.1021/jo062586z | |
 methylamine (in water) | water | N Parameter: 13.85 sN Parameter: 0.53 | J. Org. Chem. 2007, 72, 3679-3688 DOI: 10.1021/jo062586z | |
 ethylamine (in water) | water | N Parameter: 12.87 sN Parameter: 0.58 | J. Org. Chem. 2007, 72, 3679-3688 DOI: 10.1021/jo062586z | |
 isopropylamine (in water) | water | N Parameter: 12.00 sN Parameter: 0.56 | J. Org. Chem. 2007, 72, 3679-3688 DOI: 10.1021/jo062586z | |
 tert-butylamine (in water) | water | N Parameter: 10.48 sN Parameter: 0.65 | J. Org. Chem. 2007, 72, 3679-3688 DOI: 10.1021/jo062586z | |
 dimethylamine (in water) | water | N Parameter: 17.12 sN Parameter: 0.50 | J. Org. Chem. 2007, 72, 3679-3688 DOI: 10.1021/jo062586z | |
 diethylamine (in water) | water | N Parameter: 14.68 sN Parameter: 0.53 | J. Org. Chem. 2007, 72, 3679-3688 DOI: 10.1021/jo062586z | |
 pyrrolidine (in water) | water | N Parameter: 17.21 sN Parameter: 0.49 | J. Org. Chem. 2007, 72, 3679-3688 DOI: 10.1021/jo062586z | |
 piperidine (in water) | water | N Parameter: 18.13 sN Parameter: 0.44 | J. Org. Chem. 2007, 72, 3679-3688 DOI: 10.1021/jo062586z | |
 morpholine (in water) | water | N Parameter: 15.62 sN Parameter: 0.54 | J. Org. Chem. 2007, 72, 3679-3688 DOI: 10.1021/jo062586z | |
 piperazine (in water) | water | N Parameter: 17.22 sN Parameter: 0.50 | J. Org. Chem. 2007, 72, 3679-3688 DOI: 10.1021/jo062586z | |
 perhydroazepine (in water) | water | N Parameter: 18.29 sN Parameter: 0.46 | J. Org. Chem. 2007, 72, 3679-3688 DOI: 10.1021/jo062586z | |
 glycinenitrile (in water) | water | N Parameter: 10.80 sN Parameter: 0.61 | J. Org. Chem. 2007, 72, 3679-3688 DOI: 10.1021/jo062586z | |
 ethanolamine (in water) | water | N Parameter: 12.61 sN Parameter: 0.58 | J. Org. Chem. 2007, 72, 3679-3688 DOI: 10.1021/jo062586z | |
 ethylenediamine (in water) | water | N Parameter: 13.28 sN Parameter: 0.58 | J. Org. Chem. 2007, 72, 3679-3688 DOI: 10.1021/jo062586z | |
 propargylamine (in water) | water | N Parameter: 12.29 sN Parameter: 0.59 | J. Org. Chem. 2007, 72, 3679-3688 DOI: 10.1021/jo062586z | |
 allylamine (in water) | water | N Parameter: 13.21 sN Parameter: 0.54 | J. Org. Chem. 2007, 72, 3679-3688 DOI: 10.1021/jo062586z | |
 benzylamine (in water) | water | N Parameter: 13.44 sN Parameter: 0.55 | J. Org. Chem. 2007, 72, 3679-3688 DOI: 10.1021/jo062586z | |
 4-chloroaniline (in MeCN) | MeCN | N Parameter: 12.92 sN Parameter: 0.60 | J. Org. Chem. 2007, 72, 3679-3688 DOI: 10.1021/jo062586z | |
 aniline (in water) | water | N Parameter: 12.99 sN Parameter: 0.73 | J. Org. Chem. 2007, 72, 3679-3688 DOI: 10.1021/jo062586z | |
 aniline (in MeCN) | MeCN | N Parameter: 12.64 sN Parameter: 0.68 | J. Org. Chem. 2007, 72, 3679-3688 DOI: 10.1021/jo062586z | |
 p-toluidine (in water) | water | N Parameter: 13.00 sN Parameter: 0.79 | J. Org. Chem. 2007, 72, 3679-3688 DOI: 10.1021/jo062586z | |
 p-toluidine (in MeCN) | MeCN | N Parameter: 13.19 sN Parameter: 0.69 | J. Org. Chem. 2007, 72, 3679-3688 DOI: 10.1021/jo062586z | |
 4-methoxyaniline (in water) | water | N Parameter: 14.28 sN Parameter: 0.68 | ChemPlusChem 2015, 80, 1673-1679 DOI: 10.1002/cplu.201500246 | |
 4-methoxyaniline (in MeCN) | MeCN | N Parameter: 13.42 sN Parameter: 0.73 | J. Org. Chem. 2007, 72, 3679-3688 DOI: 10.1021/jo062586z | |
 diethanolamine (in water) | water | N Parameter: 13.00 sN Parameter: 0.61 | J. Org. Chem. 2007, 72, 3679-3688 DOI: 10.1021/jo062586z | |
 N-methyl-glycinenitrile (in water) | water | N Parameter: 13.50 sN Parameter: 0.59 | J. Org. Chem. 2007, 72, 3679-3688 DOI: 10.1021/jo062586z | |
 methyl glycinate (in water) | water | N Parameter: 12.08 sN Parameter: 0.60 | J. Org. Chem. 2007, 72, 3679-3688 DOI: 10.1021/jo062586z | |
 glycineamide (in water) | water | N Parameter: 12.29 sN Parameter: 0.58 | J. Org. Chem. 2007, 72, 3679-3688 DOI: 10.1021/jo062586z | |
 1,3-diaminopropane (in water) | water | N Parameter: 14.02 sN Parameter: 0.54 | J. Org. Chem. 2007, 72, 3679-3688 DOI: 10.1021/jo062586z | |
 DABCO (in MeCN) | MeCN | N Parameter: 18.80 sN Parameter: 0.70 | Angew. Chem. Int. Ed. 2007, 46, 6176-6179 DOI: 10.1002/anie.200701489 | |
 quinuclidine (in MeCN) | MeCN | N Parameter: 20.54 sN Parameter: 0.60 | Angew. Chem. Int. Ed. 2007, 46, 6176-6179 DOI: 10.1002/anie.200701489 | |
 glycine (anionic, in water) | water | N Parameter: 13.51 sN Parameter: 0.58 | Org. Biomol. Chem. 2007, 5, 3814-3820 DOI: 10.1039/b713778h | |
 alanine (anionic, in water) | water | N Parameter: 13.01 sN Parameter: 0.58 | Org. Biomol. Chem. 2007, 5, 3814-3820 DOI: 10.1039/b713778h | |
 valine (anionic, in water) | water | N Parameter: 13.65 sN Parameter: 0.57 | Org. Biomol. Chem. 2007, 5, 3814-3820 DOI: 10.1039/b713778h | |
 leucine (anionic, in water) | water | N Parameter: 14.01 sN Parameter: 0.52 | Org. Biomol. Chem. 2007, 5, 3814-3820 DOI: 10.1039/b713778h | |
 phenylalanine (anionic, in water) | water | N Parameter: 14.12 sN Parameter: 0.53 | Org. Biomol. Chem. 2007, 5, 3814-3820 DOI: 10.1039/b713778h | |
 proline (anionic, in water) | water | N Parameter: 18.08 sN Parameter: 0.50 | Org. Biomol. Chem. 2007, 5, 3814-3820 DOI: 10.1039/b713778h | |
 serine (anionic, in water) | water | N Parameter: 13.16 sN Parameter: 0.55 | Org. Biomol. Chem. 2007, 5, 3814-3820 DOI: 10.1039/b713778h | |
 threonine (anionic, in water) | water | N Parameter: 12.69 sN Parameter: 0.60 | Org. Biomol. Chem. 2007, 5, 3814-3820 DOI: 10.1039/b713778h | |
 asparagine (anionic, in water) | water | N Parameter: 13.03 sN Parameter: 0.53 | Org. Biomol. Chem. 2007, 5, 3814-3820 DOI: 10.1039/b713778h | |
 glutamine (anionic, in water) | water | N Parameter: 13.45 sN Parameter: 0.54 | Org. Biomol. Chem. 2007, 5, 3814-3820 DOI: 10.1039/b713778h | |
 arginine (betaine, in water) | water | N Parameter: 12.96 sN Parameter: 0.57 | Org. Biomol. Chem. 2007, 5, 3814-3820 DOI: 10.1039/b713778h | |
 histidine (anionic, in water) | water | N Parameter: 13.83 sN Parameter: 0.54 | Org. Biomol. Chem. 2007, 5, 3814-3820 DOI: 10.1039/b713778h | |
 aspartate (dianionic, in water) | water | N Parameter: 13.81 sN Parameter: 0.53 | Org. Biomol. Chem. 2007, 5, 3814-3820 DOI: 10.1039/b713778h | |
 glutamate (dianionic, in water) | water | N Parameter: 13.96 sN Parameter: 0.54 | Org. Biomol. Chem. 2007, 5, 3814-3820 DOI: 10.1039/b713778h | |
 cysteine (dianionic, in water, S-nucleophile!) | water | N Parameter: 23.43 sN Parameter: 0.42 | Org. Biomol. Chem. 2007, 5, 3814-3820 DOI: 10.1039/b713778h | |
 methionine (anionic, in water) | water | N Parameter: 13.16 sN Parameter: 0.58 | Org. Biomol. Chem. 2007, 5, 3814-3820 DOI: 10.1039/b713778h | |
 beta-alanine (anionic, in water) | water | N Parameter: 13.26 sN Parameter: 0.58 | Org. Biomol. Chem. 2007, 5, 3814-3820 DOI: 10.1039/b713778h | |
 gamma-aminobutyric acid (anionic, in water) | water | N Parameter: 13.55 sN Parameter: 0.56 | Org. Biomol. Chem. 2007, 5, 3814-3820 DOI: 10.1039/b713778h | |
 glycylglycine (anionic, in water) | water | N Parameter: 12.91 sN Parameter: 0.59 | Org. Biomol. Chem. 2007, 5, 3814-3820 DOI: 10.1039/b713778h | |
 glycylglycylglycine (anionic, in water) | water | N Parameter: 12.26 sN Parameter: 0.63 | Org. Biomol. Chem. 2007, 5, 3814-3820 DOI: 10.1039/b713778h | |
 DBU (in MeCN) | MeCN | N Parameter: 15.29 sN Parameter: 0.70 | Chem. Commun. 2008, , 1792-1794 DOI: 10.1039/b801811a | |
 DBN (in MeCN) | MeCN | N Parameter: 16.28 sN Parameter: 0.67 | Chem. Commun. 2008, , 1792-1794 DOI: 10.1039/b801811a | |
 cyanate (in MeCN) | MeCN | N Parameter: 13.60 sN Parameter: 0.84 | Chem. Eur. J. 2008, 14, 3866-3868 DOI: 10.1002/chem.200800314 | |
 2-aminobutan-1-ol (in DMSO) | DMSO | N Parameter: 14.39 sN Parameter: 0.67 | J. Am. Chem. Soc. 2009, 131, 11392-11401 DOI: 10.1021/ja903207b | |
 benzylamine (in DMSO) | DMSO | N Parameter: 15.28 sN Parameter: 0.65 | J. Am. Chem. Soc. 2009, 131, 11392-11401 DOI: 10.1021/ja903207b | |
 1-aminopropan-2-ol (in DMSO) | DMSO | N Parameter: 15.47 sN Parameter: 0.65 | J. Am. Chem. Soc. 2009, 131, 11392-11401 DOI: 10.1021/ja903207b | |
 diethanolamine (in DMSO) | DMSO | N Parameter: 15.51 sN Parameter: 0.70 | J. Am. Chem. Soc. 2009, 131, 11392-11401 DOI: 10.1021/ja903207b | |
 ethanolamine (in DMSO) | DMSO | N Parameter: 16.07 sN Parameter: 0.61 | J. Am. Chem. Soc. 2009, 131, 11392-11401 DOI: 10.1021/ja903207b | |
 6-methoxy-quinoline (in MeCN) | MeCN | N Parameter: 10.86 sN Parameter: 0.66 | J. Org. Chem. 2009, 74, 7157-7164 DOI: 10.1021/jo901670w | |
 4-methyl-quinoline alias lepidine (in MeCN) | MeCN | N Parameter: 11.60 sN Parameter: 0.62 | J. Org. Chem. 2009, 74, 7157-7164 DOI: 10.1021/jo901670w | |
 2-(naphthylmethyl)quinuclidine (in MeCN) | MeCN | N Parameter: 15.66 sN Parameter: 0.62 | J. Org. Chem. 2009, 74, 7157-7164 DOI: 10.1021/jo901670w | |
 quinine | dichloromethane | N Parameter: 10.46 sN Parameter: 0.75 | J. Org. Chem. 2009, 74, 7157-7164 DOI: 10.1021/jo901670w | |
 quinidine | dichloromethane | N Parameter: 10.54 sN Parameter: 0.74 | J. Org. Chem. 2009, 74, 7157-7164 DOI: 10.1021/jo901670w | |
 2,2,2-trifluoroethylamine (in MeCN) | MeCN | N Parameter: 10.13 sN Parameter: 0.75 | Eur. J. Org. Chem. 2009, , 6379-6385 DOI: 10.1002/ejoc.200900925 | |
 tert-butylamine (in MeCN) | MeCN | N Parameter: 12.35 sN Parameter: 0.72 | Eur. J. Org. Chem. 2009, , 6379-6385 DOI: 10.1002/ejoc.200900925 | |
 isopropylamine (in MeCN) | MeCN | N Parameter: 13.77 sN Parameter: 0.70 | Eur. J. Org. Chem. 2009, , 6379-6385 DOI: 10.1002/ejoc.200900925 | |
 ethanolamine (in MeCN) | MeCN | N Parameter: 14.11 sN Parameter: 0.71 | Eur. J. Org. Chem. 2009, , 6379-6385 DOI: 10.1002/ejoc.200900925 | |
 benzylamine (in MeCN) | MeCN | N Parameter: 14.29 sN Parameter: 0.67 | Eur. J. Org. Chem. 2009, , 6379-6385 DOI: 10.1002/ejoc.200900925 | |
 allylamine (in MeCN) | MeCN | N Parameter: 14.37 sN Parameter: 0.66 | Eur. J. Org. Chem. 2009, , 6379-6385 DOI: 10.1002/ejoc.200900925 | |
 n-propylamine (in MeCN) | MeCN | N Parameter: 15.11 sN Parameter: 0.63 | Eur. J. Org. Chem. 2009, , 6379-6385 DOI: 10.1002/ejoc.200900925 | |
 n-butylamine (in MeCN) | MeCN | N Parameter: 15.27 sN Parameter: 0.63 | Eur. J. Org. Chem. 2009, , 6379-6385 DOI: 10.1002/ejoc.200900925 | |
 dipropylamine (in MeCN) | MeCN | N Parameter: 14.51 sN Parameter: 0.80 | Eur. J. Org. Chem. 2009, , 6379-6385 DOI: 10.1002/ejoc.200900925 | |
 diethylamine (in MeCN) | MeCN | N Parameter: 15.10 sN Parameter: 0.73 | Eur. J. Org. Chem. 2009, , 6379-6385 DOI: 10.1002/ejoc.200900925 | |
 morpholine (in MeCN) | MeCN | N Parameter: 15.65 sN Parameter: 0.74 | Eur. J. Org. Chem. 2009, , 6379-6385 DOI: 10.1002/ejoc.200900925 | |
 piperidine (in MeCN) | MeCN | N Parameter: 17.35 sN Parameter: 0.68 | Eur. J. Org. Chem. 2009, , 6379-6385 DOI: 10.1002/ejoc.200900925 | |
 di(methoxyethyl)amine (in MeCN) | MeCN | N Parameter: 13.24 sN Parameter: 0.93 | Eur. J. Org. Chem. 2009, , 6379-6385 DOI: 10.1002/ejoc.200900925 | |
 imidazole (in MeCN) | MeCN | N Parameter: 11.47 sN Parameter: 0.79 | Org. Biomol. Chem. 2010, 8, 1929-1935 DOI: 10.1039/c000965b | |
 imidazole (in DMSO) | DMSO | N Parameter: 11.58 sN Parameter: 0.79 | Org. Biomol. Chem. 2010, 8, 1929-1935 DOI: 10.1039/c000965b | |
 imidazole (in water) | water | N Parameter: 9.63 sN Parameter: 0.57 | Org. Biomol. Chem. 2010, 8, 1929-1935 DOI: 10.1039/c000965b | |
 1-methyl-imidazole (in MeCN) | MeCN | N Parameter: 11.90 sN Parameter: 0.73 | Org. Biomol. Chem. 2010, 8, 1929-1935 DOI: 10.1039/c000965b | |
 1-methyl-imidazole (in water) | water | N Parameter: 9.91 sN Parameter: 0.55 | Org. Biomol. Chem. 2010, 8, 1929-1935 DOI: 10.1039/c000965b | |
 1-phenyl-imidazole (in MeCN) | MeCN | N Parameter: 11.31 sN Parameter: 0.67 | Org. Biomol. Chem. 2010, 8, 1929-1935 DOI: 10.1039/c000965b | |
 2-methyl-imidazole (in MeCN) | MeCN | N Parameter: 11.74 sN Parameter: 0.76 | Org. Biomol. Chem. 2010, 8, 1929-1935 DOI: 10.1039/c000965b | |
 2-methyl-imidazole (in water) | water | N Parameter: 9.45 sN Parameter: 0.54 | Org. Biomol. Chem. 2010, 8, 1929-1935 DOI: 10.1039/c000965b | |
 4-methyl-imidazole (in MeCN) | MeCN | N Parameter: 11.79 sN Parameter: 0.77 | Org. Biomol. Chem. 2010, 8, 1929-1935 DOI: 10.1039/c000965b | |
 2,4-dimethyl-imidazole (in MeCN) | MeCN | N Parameter: 11.51 sN Parameter: 0.84 | Org. Biomol. Chem. 2010, 8, 1929-1935 DOI: 10.1039/c000965b | |
 1-(trimethylsilyl)-imidazole (in MeCN) | MeCN | N Parameter: 11.43 sN Parameter: 0.79 | Org. Biomol. Chem. 2010, 8, 1929-1935 DOI: 10.1039/c000965b | |
 benzimidazole (in DMSO) | DMSO | N Parameter: 10.50 sN Parameter: 0.79 | Org. Biomol. Chem. 2010, 8, 1929-1935 DOI: 10.1039/c000965b | |
 1-methyl-benzimidazole (in MeCN) | MeCN | N Parameter: 10.37 sN Parameter: 0.82 | Org. Biomol. Chem. 2010, 8, 1929-1935 DOI: 10.1039/c000965b | |
 2-methyl-benzimidazole (in DMSO) | DMSO | N Parameter: 10.02 sN Parameter: 0.85 | Org. Biomol. Chem. 2010, 8, 1929-1935 DOI: 10.1039/c000965b | |
 5-methyl-benzimidazole (in DMSO) | DMSO | N Parameter: 10.69 sN Parameter: 0.79 | Org. Biomol. Chem. 2010, 8, 1929-1935 DOI: 10.1039/c000965b | |
 2,5-dimethyl-benzimidazole (in DMSO) | DMSO | N Parameter: 10.21 sN Parameter: 0.85 | Org. Biomol. Chem. 2010, 8, 1929-1935 DOI: 10.1039/c000965b | |
 5,6-dimethyl-benzimidazole (in DMSO) | DMSO | N Parameter: 11.08 sN Parameter: 0.71 | Org. Biomol. Chem. 2010, 8, 1929-1935 DOI: 10.1039/c000965b | |
 N-methyl-pyrrolidine (in MeCN) | MeCN | N Parameter: 20.59 sN Parameter: 0.52 | J. Phys. Org. Chem. 2010, 23, 1029-1035 DOI: 10.1002/poc.1707 | |
 N-methyl-piperidine (in MeCN) | MeCN | N Parameter: 18.72 sN Parameter: 0.52 | J. Phys. Org. Chem. 2010, 23, 1029-1035 DOI: 10.1002/poc.1707 | |
 phthalimide anion (in DMSO) | DMSO | N Parameter: 15.52 sN Parameter: 0.67 | J. Org. Chem. 2010, 75, 5250-5258 DOI: 10.1021/jo1009883 | |
 succinimide anion (in DMSO) | DMSO | N Parameter: 16.03 sN Parameter: 0.66 | J. Org. Chem. 2010, 75, 5250-5258 DOI: 10.1021/jo1009883 | |
 maleimide anion (in DMSO) | DMSO | N Parameter: 14.87 sN Parameter: 0.76 | J. Org. Chem. 2010, 75, 5250-5258 DOI: 10.1021/jo1009883 | |
 trifluoroacetamide anion (in DMSO) | DMSO | N Parameter: 15.81 sN Parameter: 0.64 | J. Org. Chem. 2010, 75, 5250-5258 DOI: 10.1021/jo1009883 | |
 N-methyl-trifluoroacetamide anion (in DMSO) | DMSO | N Parameter: 15.70 sN Parameter: 0.71 | J. Org. Chem. 2010, 75, 5250-5258 DOI: 10.1021/jo1009883 | |
 4,4-dimethyl-glutarimide anion (in DMSO) | DMSO | N Parameter: 17.52 sN Parameter: 0.63 | J. Org. Chem. 2010, 75, 5250-5258 DOI: 10.1021/jo1009883 | |
 diacetamide anion (in DMSO) | DMSO | N Parameter: 16.05 sN Parameter: 0.70 | J. Org. Chem. 2010, 75, 5250-5258 DOI: 10.1021/jo1009883 | |
 cyanamide anion (in DMSO) | DMSO | N Parameter: 20.33 sN Parameter: 0.64 | J. Org. Chem. 2010, 75, 5250-5258 DOI: 10.1021/jo1009883 | |
 p-toluenesulfonamide anion (in DMSO) | DMSO | N Parameter: 17.14 sN Parameter: 0.60 | J. Org. Chem. 2010, 75, 5250-5258 DOI: 10.1021/jo1009883 | |
 methanesulfonamide anion (in DMSO) | DMSO | N Parameter: 18.61 sN Parameter: 0.53 | J. Org. Chem. 2010, 75, 5250-5258 DOI: 10.1021/jo1009883 | |
 hydantoin anion (in DMSO) | DMSO | N Parameter: 17.52 sN Parameter: 0.55 | J. Org. Chem. 2010, 75, 5250-5258 DOI: 10.1021/jo1009883 | |
 1,3-oxazolidin-2-one anion (in DMSO) | DMSO | N Parameter: 22.40 sN Parameter: 0.59 | J. Org. Chem. 2010, 75, 5250-5258 DOI: 10.1021/jo1009883 | |
 (S)-4-benzyl-1,3-oxazolidin-2-one anion (in DMSO) | DMSO | N Parameter: 22.67 sN Parameter: 0.54 | J. Org. Chem. 2010, 75, 5250-5258 DOI: 10.1021/jo1009883 | |
 ethyl N-acetylcarbamate anion (in DMSO) | DMSO | N Parameter: 15.99 sN Parameter: 0.70 | J. Org. Chem. 2010, 75, 5250-5258 DOI: 10.1021/jo1009883 | |
 2-pyridone anion (in DMSO) | DMSO | N Parameter: 19.91 sN Parameter: 0.60 | J. Am. Chem. Soc. 2010, 132, 15380-15389 DOI: 10.1021/ja106962u | |
 2-pyridone anion (in MeCN) | MeCN | N Parameter: 20.11 sN Parameter: 0.57 | J. Am. Chem. Soc. 2010, 132, 15380-15389 DOI: 10.1021/ja106962u | |
 2-pyridone anion (in water) | water | N Parameter: 12.47 sN Parameter: 0.52 | J. Am. Chem. Soc. 2010, 132, 15380-15389 DOI: 10.1021/ja106962u | |
 4-pyridone anion (in DMSO) | DMSO | N Parameter: 18.97 sN Parameter: 0.62 | J. Am. Chem. Soc. 2010, 132, 15380-15389 DOI: 10.1021/ja106962u | |
 4-pyridone anion (in MeCN) | MeCN | N Parameter: 20.22 sN Parameter: 0.49 | J. Am. Chem. Soc. 2010, 132, 15380-15389 DOI: 10.1021/ja106962u | |
 4-pyridone anion (in water) | water | N Parameter: 14.76 sN Parameter: 0.48 | J. Am. Chem. Soc. 2010, 132, 15380-15389 DOI: 10.1021/ja106962u | |
 saccharin anion (in MeCN) | MeCN | N Parameter: 10.78 sN Parameter: 0.89 | J. Org. Chem. 2010, 75, 5250-5258 DOI: 10.1021/jo1009883 | |
 2-Me super-dmap (in MeCN) | MeCN | N Parameter: 16.65 sN Parameter: 0.58 | Org. Lett. 2011, 13, 530-533 DOI: 10.1021/ol1029589 | |
 2-Et super-dmap (in MeCN) | MeCN | N Parameter: 16.81 sN Parameter: 0.60 | Org. Lett. 2011, 13, 530-533 DOI: 10.1021/ol1029589 | |
 2-Bn super-dmap (in MeCN) | MeCN | N Parameter: 17.69 sN Parameter: 0.57 | Org. Lett. 2011, 13, 530-533 DOI: 10.1021/ol1029589 | |
 2-Ac super-dmap (in MeCN) | MeCN | N Parameter: 15.39 sN Parameter: 0.60 | Org. Lett. 2011, 13, 530-533 DOI: 10.1021/ol1029589 | |
 2-Bz super-dmap (in MeCN) | MeCN | N Parameter: 14.19 sN Parameter: 0.67 | Org. Lett. 2011, 13, 530-533 DOI: 10.1021/ol1029589 | |
 3,5,6,7-tetrahydro-2H-imidazo[2,1-b][1,3]thiazine | dichloromethane | N Parameter: 13.00 sN Parameter: 0.83 | J. Org. Chem. 2011, 76, 5104-5112 DOI: 10.1021/jo200803x | |
 2,3,5,6-tetrahydroimidazo[2,1-b]thiazole | dichloromethane | N Parameter: 12.98 sN Parameter: 0.81 | J. Org. Chem. 2011, 76, 5104-5112 DOI: 10.1021/jo200803x | |
 2,3-dihydrobenzo[d]imidazo[2,1-b]thiazole | dichloromethane | N Parameter: 13.42 sN Parameter: 0.73 | J. Org. Chem. 2011, 76, 5104-5112 DOI: 10.1021/jo200803x | |
 2,3,4,6,7,8-hexahydropyrimido[2,1-b][1,3]thiazine | dichloromethane | N Parameter: 14.10 sN Parameter: 0.82 | J. Org. Chem. 2011, 76, 5104-5112 DOI: 10.1021/jo200803x | |
 THTP (3,5,6,7-tetrahydro-2H-thiazolo[3,2-a]pyrimidine) | dichloromethane | N Parameter: 14.45 sN Parameter: 0.78 | J. Org. Chem. 2011, 76, 5104-5112 DOI: 10.1021/jo200803x | |
 DHPB (in CH2Cl2) | dichloromethane | N Parameter: 13.86 sN Parameter: 0.78 | J. Org. Chem. 2011, 76, 5104-5112 DOI: 10.1021/jo200803x | |
 HBTM (in CH2Cl2) | dichloromethane | N Parameter: 13.45 sN Parameter: 0.72 | J. Org. Chem. 2011, 76, 5104-5112 DOI: 10.1021/jo200803x | |
 HyperBTM (in CH2Cl2) | dichloromethane | N Parameter: 14.96 sN Parameter: 0.64 | J. Org. Chem. 2011, 76, 5104-5112 DOI: 10.1021/jo200803x | |
 (R)-2-benzhydryl-3,4-dihydro-2H-benzo[4,5]thiazolo[3,2-a]pyrimidine | dichloromethane | N Parameter: 15.30 sN Parameter: 0.55 | J. Org. Chem. 2011, 76, 5104-5112 DOI: 10.1021/jo200803x | |
 (R)-2-isopropyl-3,4-dihydro-2H-benzo[4,5]thiazolo[3,2-a]pyrimidine | dichloromethane | N Parameter: 16.50 sN Parameter: 0.48 | J. Org. Chem. 2011, 76, 5104-5112 DOI: 10.1021/jo200803x | |
 (R)-2-(tert-butyl)-3,4-dihydro-2H-benzo[4,5]thiazolo[3,2-a]pyrimidine | dichloromethane | N Parameter: 12.95 sN Parameter: 0.58 | J. Org. Chem. 2011, 76, 5104-5112 DOI: 10.1021/jo200803x | |
 hydrazine (in MeCN) | MeCN | N Parameter: 16.45 sN Parameter: 0.56 | Angew. Chem. Int. Ed. 2012, 51, 1353-1356 DOI: 10.1002/anie.201107315 | |
 1,1-dimethylhydrazine (in MeCN) | MeCN | N Parameter: 11.72 sN Parameter: 0.73 | Angew. Chem. Int. Ed. 2012, 51, 1353-1356 DOI: 10.1002/anie.201107315 | |
 trimethylhydrazine (in MeCN) | MeCN | N Parameter: 12.43 sN Parameter: 0.75 | Angew. Chem. Int. Ed. 2012, 51, 1353-1356 DOI: 10.1002/anie.201107315 | |
 1,1-dimethylhydrazine (in MeCN) | MeCN | N Parameter: 22.41 sN Parameter: 0.45 | Angew. Chem. Int. Ed. 2012, 51, 1353-1356 DOI: 10.1002/anie.201107315 | |
 trimethylhydrazine (in MeCN) | MeCN | N Parameter: 17.75 sN Parameter: 0.53 | Angew. Chem. Int. Ed. 2012, 51, 1353-1356 DOI: 10.1002/anie.201107315 | |
 imidazole anion (in DMSO) | DMSO | N Parameter: 21.09 sN Parameter: 0.51 | Chem. Eur. J. 2012, 18, 127-137 DOI: 10.1002/chem.201102411 | |
 2-methyl-imidazole anion (in DMSO) | DMSO | N Parameter: 21.03 sN Parameter: 0.50 | Chem. Eur. J. 2012, 18, 127-137 DOI: 10.1002/chem.201102411 | |
 4-methyl-imidazole anion (in DMSO) | DMSO | N Parameter: 21.29 sN Parameter: 0.51 | Chem. Eur. J. 2012, 18, 127-137 DOI: 10.1002/chem.201102411 | |
 2,4-dimethyl-imidazole anion (in DMSO) | DMSO | N Parameter: 20.69 sN Parameter: 0.60 | Chem. Eur. J. 2012, 18, 127-137 DOI: 10.1002/chem.201102411 | |
 4-nitro-imidazole anion (in DMSO) | DMSO | N Parameter: 14.81 sN Parameter: 0.71 | Chem. Eur. J. 2012, 18, 127-137 DOI: 10.1002/chem.201102411 | |
 2-formyl-imidazole anion (in DMSO) | DMSO | N Parameter: 16.06 sN Parameter: 0.68 | Chem. Eur. J. 2012, 18, 127-137 DOI: 10.1002/chem.201102411 | |
 4-formyl-imidazole anion (in DMSO) | DMSO | N Parameter: 16.40 sN Parameter: 0.67 | Chem. Eur. J. 2012, 18, 127-137 DOI: 10.1002/chem.201102411 | |
 benzimidazole anion (in DMSO) | DMSO | N Parameter: 19.13 sN Parameter: 0.55 | Chem. Eur. J. 2012, 18, 127-137 DOI: 10.1002/chem.201102411 | |
 benzotriazole anion (in DMSO) | DMSO | N Parameter: 16.29 sN Parameter: 0.65 | Chem. Eur. J. 2012, 18, 127-137 DOI: 10.1002/chem.201102411 | |
 purine anion (in DMSO) | DMSO | N Parameter: 15.03 sN Parameter: 0.77 | Chem. Eur. J. 2012, 18, 127-137 DOI: 10.1002/chem.201102411 | |
 theophylline anion (in DMSO) | DMSO | N Parameter: 14.78 sN Parameter: 0.71 | Chem. Eur. J. 2012, 18, 127-137 DOI: 10.1002/chem.201102411 | |
 adenine anion (in DMSO) | DMSO | N Parameter: 18.00 sN Parameter: 0.55 | Chem. Eur. J. 2012, 18, 127-137 DOI: 10.1002/chem.201102411 | |
 uracil anion (in DMSO) | DMSO | N Parameter: 17.04 sN Parameter: 0.63 | Chem. Eur. J. 2012, 18, 127-137 DOI: 10.1002/chem.201102411 | |
 thymine anion (in DMSO) | DMSO | N Parameter: 17.63 sN Parameter: 0.62 | Chem. Eur. J. 2012, 18, 127-137 DOI: 10.1002/chem.201102411 | |
 1-methyl uracil anion (in DMSO) | DMSO | N Parameter: 16.36 sN Parameter: 0.69 | Chem. Eur. J. 2012, 18, 127-137 DOI: 10.1002/chem.201102411 | |
 4-nitro-imidazole anion (in water) | water | N Parameter: 11.37 sN Parameter: 0.53 | Chem. Eur. J. 2012, 18, 127-137 DOI: 10.1002/chem.201102411 | |
 2-formyl-imidazole anion (in water) | water | N Parameter: 11.07 sN Parameter: 0.50 | Chem. Eur. J. 2012, 18, 127-137 DOI: 10.1002/chem.201102411 | |
 benzotriazole anion (in water) | water | N Parameter: 11.52 sN Parameter: 0.67 | Chem. Eur. J. 2012, 18, 127-137 DOI: 10.1002/chem.201102411 | |
 purine anion (in water) | water | N Parameter: 11.00 sN Parameter: 0.54 | Chem. Eur. J. 2012, 18, 127-137 DOI: 10.1002/chem.201102411 | |
 theophylline anion (in water) | water | N Parameter: 10.06 sN Parameter: 0.71 | Chem. Eur. J. 2012, 18, 127-137 DOI: 10.1002/chem.201102411 | |
 adenine anion (in water) | water | N Parameter: 10.93 sN Parameter: 0.62 | Chem. Eur. J. 2012, 18, 127-137 DOI: 10.1002/chem.201102411 | |
 guanine anion (in water) | water | N Parameter: 11.63 sN Parameter: 0.59 | Chem. Eur. J. 2012, 18, 127-137 DOI: 10.1002/chem.201102411 | |
 9-methyl guanine anion (in water) | water | N Parameter: 10.77 sN Parameter: 0.65 | Chem. Eur. J. 2012, 18, 127-137 DOI: 10.1002/chem.201102411 | |
 guanosine anion (in water) | water | N Parameter: 12.09 sN Parameter: 0.52 | Chem. Eur. J. 2012, 18, 127-137 DOI: 10.1002/chem.201102411 | |
 uracil anion (in water) | water | N Parameter: 10.75 sN Parameter: 0.53 | Chem. Eur. J. 2012, 18, 127-137 DOI: 10.1002/chem.201102411 | |
 thymine anion (in water) | water | N Parameter: 11.17 sN Parameter: 0.51 | Chem. Eur. J. 2012, 18, 127-137 DOI: 10.1002/chem.201102411 | |
 1-methyl uracil anion (in water) | water | N Parameter: 8.54 sN Parameter: 0.77 | Chem. Eur. J. 2012, 18, 127-137 DOI: 10.1002/chem.201102411 | |
 1,1,3,3-tetramethylguanidine | dichloromethane | N Parameter: 13.58 sN Parameter: 0.77 | ChemCatChem 2012, 4, 993-999 DOI: 10.1002/cctc.201200143 | |
 2-benzyl-1,1,3,3-tetramethylguanidine | dichloromethane | N Parameter: 14.36 sN Parameter: 0.79 | ChemCatChem 2012, 4, 993-999 DOI: 10.1002/cctc.201200143 | |
 1,3-dimethylimidazolidin-2-imine | dichloromethane | N Parameter: 12.46 sN Parameter: 0.87 | ChemCatChem 2012, 4, 993-999 DOI: 10.1002/cctc.201200143 | |
 N-(1,3-dimethylimidazolidin-2-ylidene)-1-phenylmethanamine | dichloromethane | N Parameter: 14.00 sN Parameter: 0.70 | ChemCatChem 2012, 4, 993-999 DOI: 10.1002/cctc.201200143 | |
 2,3,5,6-tetrahydro-1H-imidazo[1,2-a]imidazole (TBO) | dichloromethane | N Parameter: 14.44 sN Parameter: 0.79 | ChemCatChem 2012, 4, 993-999 DOI: 10.1002/cctc.201200143 | |
 1,2,3,5,6,7-hexahydroimidazo[1,2-a]pyrimidine (TBN) | dichloromethane | N Parameter: 16.15 sN Parameter: 0.73 | ChemCatChem 2012, 4, 993-999 DOI: 10.1002/cctc.201200143 | |
 1-methyl-2,3,4,6,7,8-hexahydro-1H-pyrimido[1,2-a]pyrimidine (MeTBD) | dichloromethane | N Parameter: 14.43 sN Parameter: 0.81 | ChemCatChem 2012, 4, 993-999 DOI: 10.1002/cctc.201200143 | |
 2,3,4,6,7,8-hexahydro-1H-pyrimido[1,2-a]pyrimidine | dichloromethane | N Parameter: 16.16 sN Parameter: 0.75 | ChemCatChem 2012, 4, 993-999 DOI: 10.1002/cctc.201200143 | |
 DBN (in CH2Cl2) | dichloromethane | N Parameter: 15.50 sN Parameter: 0.76 | ChemCatChem 2012, 4, 993-999 DOI: 10.1002/cctc.201200143 | |
 4-(dimethylamino)pyridine (in THF) | THF | N Parameter: 15.90 sN Parameter: 0.66 | Angew. Chem. Int. Ed. 2011, 50, 6915-6919 DOI: 10.1002/anie.201102435 | |
 DBU (in THF) | THF | N Parameter: 16.12 sN Parameter: 0.67 | Angew. Chem. Int. Ed. 2011, 50, 6915-6919 DOI: 10.1002/anie.201102435 | |
 acetonitrile (in MeCN) | MeCN | N Parameter: 2.23 sN Parameter: 0.84 | J. Am. Chem. Soc. 2012, 134, 13902-13911 DOI: 10.1021/ja306522b | |
 4-(morpholino)pyridine (in MeCN) | MeCN | N Parameter: 14.80 sN Parameter: 0.63 | J. Phys. Chem. A 2012, 116, 8494-8499 DOI: 10.1021/jp3049247 | |
 3-bromo-4-(dimethylamino)pyridine (in MeCN) | MeCN | N Parameter: 12.96 sN Parameter: 0.67 | J. Phys. Chem. A 2012, 116, 8494-8499 DOI: 10.1021/jp3049247 | |
 4-amino-3,5-dibromo-pyridine (in MeCN) | MeCN | N Parameter: 11.11 sN Parameter: 0.75 | J. Phys. Chem. A 2012, 116, 8494-8499 DOI: 10.1021/jp3049247 | |
 4-acetamido-pyridine (in MeCN) | MeCN | N Parameter: 13.24 sN Parameter: 0.67 | J. Phys. Chem. A 2012, 116, 8494-8499 DOI: 10.1021/jp3049247 | |
 hydrazine (in water) | water | N Parameter: 13.46 sN Parameter: 0.57 | J. Org. Chem. 2012, 77, 8142-8155 DOI: 10.1021/jo301497g | |
 methylhydrazine (in water) | water | N Parameter: 17.23 sN Parameter: 0.45 | J. Org. Chem. 2012, 77, 8142-8155 DOI: 10.1021/jo301497g | |
 methylhydrazine (in MeCN) | MeCN | N Parameter: 17.73 sN Parameter: 0.58 | J. Org. Chem. 2012, 77, 8142-8155 DOI: 10.1021/jo301497g | |
 1,2-dimethylhydrazine (in MeCN) | MeCN | N Parameter: 16.15 sN Parameter: 0.68 | J. Org. Chem. 2012, 77, 8142-8155 DOI: 10.1021/jo301497g | |
 formohydrazide (in MeCN) | MeCN | N Parameter: 10.35 sN Parameter: 0.76 | J. Org. Chem. 2012, 77, 8142-8155 DOI: 10.1021/jo301497g | |
 N',N'-dimethylformohydrazide (in MeCN) | MeCN | N Parameter: 15.69 sN Parameter: 0.51 | J. Org. Chem. 2012, 77, 8142-8155 DOI: 10.1021/jo301497g | |
 tert-butyl hydrazinecarboxylate (in MeCN) | MeCN | N Parameter: 11.40 sN Parameter: 0.70 | J. Org. Chem. 2012, 77, 8142-8155 DOI: 10.1021/jo301497g | |
 benzohydrazide (in MeCN) | MeCN | N Parameter: 12.49 sN Parameter: 0.66 | J. Org. Chem. 2012, 77, 8142-8155 DOI: 10.1021/jo301497g | |
 hydroxylamine (in MeCN) | MeCN | N Parameter: 12.80 sN Parameter: 0.63 | J. Org. Chem. 2012, 77, 8142-8155 DOI: 10.1021/jo301497g | |
 N-methylhydroxylamine (in MeCN) | MeCN | N Parameter: 14.10 sN Parameter: 0.76 | J. Org. Chem. 2012, 77, 8142-8155 DOI: 10.1021/jo301497g | |
 ammonia (in MeCN) | MeCN | N Parameter: 11.39 sN Parameter: 0.69 | J. Org. Chem. 2012, 77, 8142-8155 DOI: 10.1021/jo301497g | |
 methylamine (in MeCN) | MeCN | N Parameter: 15.19 sN Parameter: 0.68 | J. Org. Chem. 2012, 77, 8142-8155 DOI: 10.1021/jo301497g | |
 dimethylamine (in MeCN) | MeCN | N Parameter: 17.96 sN Parameter: 0.63 | J. Org. Chem. 2012, 77, 8142-8155 DOI: 10.1021/jo301497g | |
 trimethylamine (in MeCN) | MeCN | N Parameter: 23.05 sN Parameter: 0.45 | J. Org. Chem. 2012, 77, 8142-8155 DOI: 10.1021/jo301497g | |
 2-methyl-4,5-dihydro-1H-imidazole (in CH2Cl2) | dichloromethane | N Parameter: 12.92 sN Parameter: 0.77 | Eur. J. Org. Chem. 2013, , 3369-3377 DOI: 10.1002/ejoc.201300213 | |
 2-phenyl-4,5-dihydro-1H-imidazole (in CH2Cl2) | dichloromethane | N Parameter: 12.31 sN Parameter: 0.77 | Eur. J. Org. Chem. 2013, , 3369-3377 DOI: 10.1002/ejoc.201300213 | |
 1-(2-methyl-4,5-dihydro-1H-imidazol-1-yl)ethanone (in CH2Cl2) | dichloromethane | N Parameter: 10.03 sN Parameter: 0.75 | Eur. J. Org. Chem. 2013, , 3369-3377 DOI: 10.1002/ejoc.201300213 | |
 1-benzyl-2-phenyl-4,5-dihydro-1H-imidazole (in CH2Cl2) | dichloromethane | N Parameter: 13.11 sN Parameter: 0.83 | Eur. J. Org. Chem. 2013, , 3369-3377 DOI: 10.1002/ejoc.201300213 | |
 2-methyl-4,5-dihydrothiazole (in CH2Cl2) | dichloromethane | N Parameter: 10.20 sN Parameter: 0.71 | Eur. J. Org. Chem. 2013, , 3369-3377 DOI: 10.1002/ejoc.201300213 | |
 2-methyl-4,5-dihydro-1,3-oxazole (in CH2Cl2) | dichloromethane | N Parameter: 9.81 sN Parameter: 0.77 | Eur. J. Org. Chem. 2013, , 3369-3377 DOI: 10.1002/ejoc.201300213 | |
 2-methyl-4,5-dihydro-3H-pyrrole (in CH2Cl2) | dichloromethane | N Parameter: 13.12 sN Parameter: 0.69 | Eur. J. Org. Chem. 2013, , 3369-3377 DOI: 10.1002/ejoc.201300213 | |
 2-methyl-1,4,5,6-tetrahydropyrimidine (in CH2Cl2) | dichloromethane | N Parameter: 15.21 sN Parameter: 0.69 | Eur. J. Org. Chem. 2013, , 3369-3377 DOI: 10.1002/ejoc.201300213 | |
 2-methyl-1,4,5,6-tetrahydropyrimidine (in MeCN) | MeCN | N Parameter: 14.43 sN Parameter: 0.79 | Eur. J. Org. Chem. 2013, , 3369-3377 DOI: 10.1002/ejoc.201300213 | |
 2-methyl-1,4,5,6-tetrahydropyrimidine (in DMSO) | DMSO | N Parameter: 14.58 sN Parameter: 0.79 | Eur. J. Org. Chem. 2013, , 3369-3377 DOI: 10.1002/ejoc.201300213 | |
 2-phenyl-1,4,5,6-tetrahydropyrimidine (in CH2Cl2) | dichloromethane | N Parameter: 14.62 sN Parameter: 0.72 | Eur. J. Org. Chem. 2013, , 3369-3377 DOI: 10.1002/ejoc.201300213 | |
 N-methyl-1-phenylmethanimine (in CH2Cl2) | dichloromethane | N Parameter: 8.60 sN Parameter: 0.77 | Z. Naturforsch. B 2013, 68b, 693-699 DOI: 10.5560/ZNB.2013-3085 | |
 N-phenylpropan-2-imine (in CH2Cl2) | dichloromethane | N Parameter: 9.53 sN Parameter: 0.85 | Z. Naturforsch. B 2013, 68b, 693-699 DOI: 10.5560/ZNB.2013-3085 | |
 N-(phenylmethyl)propan-2-imine (in CH2Cl2) | dichloromethane | N Parameter: 11.13 sN Parameter: 0.73 | Z. Naturforsch. B 2013, 68b, 693-699 DOI: 10.5560/ZNB.2013-3085 | |
 N-phenylcyclohexanimine (in CH2Cl2) | dichloromethane | N Parameter: 8.80 sN Parameter: 1.00 | Z. Naturforsch. B 2013, 68b, 693-699 DOI: 10.5560/ZNB.2013-3085 | |
 1,1-dimethyl-2-methylenehydrazine (in CH2Cl2) | dichloromethane | N Parameter: 19.31 sN Parameter: 0.46 | Angew. Chem. Int. Ed. 2013, 52, 11900-11904 DOI: 10.1002/anie.201305092 | |
 N-methylenepyrrolidin-1-amine (in CH2Cl2) | dichloromethane | N Parameter: 17.90 sN Parameter: 0.48 | Angew. Chem. Int. Ed. 2013, 52, 11900-11904 DOI: 10.1002/anie.201305092 | |
 4-(2-(fluorodiphenylmethyl)pyrrolidin-1-yl)pyridine | dichloromethane | N Parameter: 14.57 sN Parameter: 0.75 | Eur. J. Org. Chem. 2014, , 1202-1211 DOI: 10.1002/ejoc.201301730 | |
 2-phenylethylamine (in water) | water | N Parameter: 13.40 sN Parameter: 0.57 | ChemPlusChem 2015, 80, 1673-1679 DOI: 10.1002/cplu.201500246 | |
 nicotine (in CH2Cl2) | dichloromethane | N Parameter: 10.40 sN Parameter: 1.04 | J. Phys. Org. Chem. 2016, 29, 759-767 DOI: 10.1002/poc.3580 | |
 nicotine (in MeCN) | MeCN | N Parameter: 11.60 sN Parameter: 0.81 | J. Phys. Org. Chem. 2016, 29, 759-767 DOI: 10.1002/poc.3580 | |
 3-methylpyridine (in CH2Cl2) | dichloromethane | N Parameter: 10.90 sN Parameter: 0.93 | J. Phys. Org. Chem. 2016, 29, 759-767 DOI: 10.1002/poc.3580 | |
 3-methylpyridine (in MeCN) | MeCN | N Parameter: 11.50 sN Parameter: 0.80 | J. Phys. Org. Chem. 2016, 29, 759-767 DOI: 10.1002/poc.3580 | |
 1-methyl-2-phenylpyrrolidine (in CH2Cl2) | dichloromethane | N Parameter: 16.80 sN Parameter: 0.49 | J. Phys. Org. Chem. 2016, 29, 759-767 DOI: 10.1002/poc.3580 | |
 1-methyl-2-phenylpyrrolidine (in MeCN) | MeCN | N Parameter: 15.70 sN Parameter: 0.54 | J. Phys. Org. Chem. 2016, 29, 759-767 DOI: 10.1002/poc.3580 | |
 4-(morpholino)pyridine (in CH2Cl2) | dichloromethane | N Parameter: 14.59 sN Parameter: 0.69 | Eur. J. Org. Chem. 2016, , 4050-4058 DOI: 10.1002/ejoc.201600572 | |
 prolinate (in MeCN) | MeCN | N Parameter: 19.95 sN Parameter: 0.68 | J. Am. Chem. Soc. 2020, 142, 1526-1547 DOI: 10.1021/jacs.9b11877 | |
 2-methylpyrrolidine (in MeCN) | MeCN | N Parameter: 16.78 sN Parameter: 0.71 | J. Am. Chem. Soc. 2020, 142, 1526-1547 DOI: 10.1021/jacs.9b11877 | |
 pyrrolidine (inMeCN) | MeCN | N Parameter: 18.58 sN Parameter: 0.61 | J. Am. Chem. Soc. 2020, 142, 1526-1547 DOI: 10.1021/jacs.9b11877 | |
 (R)-2-isopropylpyrrolidine | MeCN | N Parameter: 16.44 sN Parameter: 0.71 | J. Am. Chem. Soc. 2020, 142, 1526-1547 DOI: 10.1021/jacs.9b11877 | |
 2,2-dimethylpyrrolidine | MeCN | N Parameter: 13.96 sN Parameter: 0.76 | J. Am. Chem. Soc. 2020, 142, 1526-1547 DOI: 10.1021/jacs.9b11877 | |
 2-benzylpyrrolidine | MeCN | N Parameter: 17.43 sN Parameter: 0.66 | J. Am. Chem. Soc. 2020, 142, 1526-1547 DOI: 10.1021/jacs.9b11877 | |
 (S)-2-benzhydrylpyrrolidine | MeCN | N Parameter: 16.61 sN Parameter: 0.67 | J. Am. Chem. Soc. 2020, 142, 1526-1547 DOI: 10.1021/jacs.9b11877 | |
 (S)-pyrrolidin-2-ylmethanamine | MeCN | N Parameter: 17.24 sN Parameter: 0.67 | J. Am. Chem. Soc. 2020, 142, 1526-1547 DOI: 10.1021/jacs.9b11877 | |
 (S)-N,N-dimethyl-1-(pyrrolidin-2-yl)methanamine | MeCN | N Parameter: 17.41 sN Parameter: 0.68 | J. Am. Chem. Soc. 2020, 142, 1526-1547 DOI: 10.1021/jacs.9b11877 | |
 (S)-1-(pyrrolidin-2-ylmethyl)pyrrolidine | MeCN | N Parameter: 18.33 sN Parameter: 0.64 | J. Am. Chem. Soc. 2020, 142, 1526-1547 DOI: 10.1021/jacs.9b11877 | |
 (S)-2-(azidomethyl)pyrrolidine | MeCN | N Parameter: 15.43 sN Parameter: 0.73 | J. Am. Chem. Soc. 2020, 142, 1526-1547 DOI: 10.1021/jacs.9b11877 | |
 (S)-pyrrolidin-2-ylmethanol | MeCN | N Parameter: 16.74 sN Parameter: 0.67 | J. Am. Chem. Soc. 2020, 142, 1526-1547 DOI: 10.1021/jacs.9b11877 | |
 (S)-2-(methoxymethyl)pyrrolidine | MeCN | N Parameter: 16.50 sN Parameter: 0.71 | J. Am. Chem. Soc. 2020, 142, 1526-1547 DOI: 10.1021/jacs.9b11877 | |
 2-(trifluoromethyl)pyrrolidine | MeCN | N Parameter: 11.34 sN Parameter: 0.73 | J. Am. Chem. Soc. 2020, 142, 1526-1547 DOI: 10.1021/jacs.9b11877 | |
 methyl L-prolinate | MeCN | N Parameter: 14.75 sN Parameter: 0.82 | J. Am. Chem. Soc. 2020, 142, 1526-1547 DOI: 10.1021/jacs.9b11877 | |
 (S)-N,N-dimethylpyrrolidine-2-carboxamide | MeCN | N Parameter: 17.61 sN Parameter: 0.67 | J. Am. Chem. Soc. 2020, 142, 1526-1547 DOI: 10.1021/jacs.9b11877 | |
 (S)-N-propylpyrrolidine-2-carboxamide | MeCN | N Parameter: 15.20 sN Parameter: 0.73 | J. Am. Chem. Soc. 2020, 142, 1526-1547 DOI: 10.1021/jacs.9b11877 | |
 (S)-2-(pyrrolidin-2-ylmethyl)isoindoline-1,3-dione | MeCN | N Parameter: 15.90 sN Parameter: 0.77 | J. Am. Chem. Soc. 2020, 142, 1526-1547 DOI: 10.1021/jacs.9b11877 | |
 (S)-4-phenyl-1-(pyrrolidin-2-ylmethyl)-1H-1,2,3-triazole | MeCN | N Parameter: 15.32 sN Parameter: 0.72 | J. Am. Chem. Soc. 2020, 142, 1526-1547 DOI: 10.1021/jacs.9b11877 | |
 (S)-1-(pyrrolidin-2-ylmethyl)-1H-imidazole | MeCN | N Parameter: 15.55 sN Parameter: 0.69 | J. Am. Chem. Soc. 2020, 142, 1526-1547 DOI: 10.1021/jacs.9b11877 | |
 (S)-3-butyl-1-(pyrrolidin-2-ylmethyl)-1H-imidazol-3-ium trifluoromethanesulfonate | MeCN | N Parameter: 13.57 sN Parameter: 0.53 | J. Am. Chem. Soc. 2020, 142, 1526-1547 DOI: 10.1021/jacs.9b11877 | |
 (S)-1-(3,5-bis(trifluoromethyl)phenyl)-3-(pyrrolidin-2-ylmethyl)thiourea | MeCN | N Parameter: 14.97 sN Parameter: 0.69 | J. Am. Chem. Soc. 2020, 142, 1526-1547 DOI: 10.1021/jacs.9b11877 | |
 (S)-1-(3,5-bis(trifluoromethyl)phenyl)-3-(pyrrolidin-2-ylmethyl)urea | MeCN | N Parameter: 17.50 sN Parameter: 0.64 | J. Am. Chem. Soc. 2020, 142, 1526-1547 DOI: 10.1021/jacs.9b11877 | |
 2-tritylpyrrolidine | MeCN | N Parameter: 9.16 sN Parameter: 1.39 | J. Am. Chem. Soc. 2020, 142, 1526-1547 DOI: 10.1021/jacs.9b11877 | |
 (S)-2-(diphenyl((trimethylsilyl)oxy)methyl)pyrrolidine | MeCN | N Parameter: 12.03 sN Parameter: 0.98 | J. Am. Chem. Soc. 2020, 142, 1526-1547 DOI: 10.1021/jacs.9b11877 | |
 (S)-diphenyl(pyrrolidin-2-yl)methanol | MeCN | N Parameter: 16.18 sN Parameter: 0.56 | J. Am. Chem. Soc. 2020, 142, 1526-1547 DOI: 10.1021/jacs.9b11877 | |
 (S)-2-(azidodiphenylmethyl)pyrrolidine | MeCN | N Parameter: 9.90 sN Parameter: 1.22 | J. Am. Chem. Soc. 2020, 142, 1526-1547 DOI: 10.1021/jacs.9b11877 | |
 2-(triphenylsilyl)pyrrolidine | MeCN | N Parameter: 14.00 sN Parameter: 0.84 | J. Am. Chem. Soc. 2020, 142, 1526-1547 DOI: 10.1021/jacs.9b11877 | |
 (S)-5-benzyl-2,2,3-trimethylimidazolidin-4-one | MeCN | N Parameter: 6.04 sN Parameter: 0.92 | J. Am. Chem. Soc. 2020, 142, 1526-1547 DOI: 10.1021/jacs.9b11877 | |
 (2S,5S)-5-benzyl-2-(tert-butyl)-3-methylimidazolidin-4-one | MeCN | N Parameter: 5.44 sN Parameter: 1.12 | J. Am. Chem. Soc. 2020, 142, 1526-1547 DOI: 10.1021/jacs.9b11877 | |
 (2S,5S)-5-benzyl-3-methyl-2-(5-methylfuran-2-yl)imidazolidin-4-one | MeCN | N Parameter: 8.76 sN Parameter: 0.89 | J. Am. Chem. Soc. 2020, 142, 1526-1547 DOI: 10.1021/jacs.9b11877 | |
 (2R,5S)-5-benzyl-3-methyl-2-(5-methylfuran-2-yl)imidazolidin-4-one | MeCN | N Parameter: 7.39 sN Parameter: 1.00 | J. Am. Chem. Soc. 2020, 142, 1526-1547 DOI: 10.1021/jacs.9b11877 | |
 TCAP (in MeCN) | MeCN | N Parameter: 15.60 sN Parameter: 0.68 | Chem. Eur. J. 2013, 19, 6435-6442 DOI: 10.1002/chem.201204452 | |
 4-pyrrolidinopyridine (in MeCN) | MeCN | N Parameter: 14.99 sN Parameter: 0.69 | Chem. Eur. J. 2013, 19, 6435-6442 DOI: 10.1002/chem.201204452 | |
 (F)2-HBTM (in CH2Cl2) | dichloromethane | N Parameter: 10.83 sN Parameter: 0.80 | ARKIVOC 2024, (4), 202312093 DOI: 10.24820/ark.5550190.p012.093 | |
 pyridine (in MeCN) | MeCN | N Parameter: 13.60 sN Parameter: 0.60 | Synthesis 2017, 49, 3495-3504 DOI: 10.1055/s-0036-1590504 | |
 2-methylpyridine (in CH2Cl2) | dichloromethane | N Parameter: 10.52 sN Parameter: 0.78 | Synthesis 2017, 49, 3495-3504 DOI: 10.1055/s-0036-1590504 | |
 2-methylpyridine (in MeCN) | MeCN | N Parameter: 10.98 sN Parameter: 0.66 | Synthesis 2017, 49, 3495-3504 DOI: 10.1055/s-0036-1590504 | |
 2,6-dimethylpyridine (in CH2Cl2) | dichloromethane | N Parameter: 9.87 sN Parameter: 0.68 | Synthesis 2017, 49, 3495-3504 DOI: 10.1055/s-0036-1590504 | |
 2,6-dimethylpyridine (in MeCN) | MeCN | N Parameter: 9.11 sN Parameter: 0.69 | Synthesis 2017, 49, 3495-3504 DOI: 10.1055/s-0036-1590504 | |
 2,4,6-trimethylpyridine (in CH2Cl2) | dichloromethane | N Parameter: 9.34 sN Parameter: 0.71 | Synthesis 2017, 49, 3495-3504 DOI: 10.1055/s-0036-1590504 | |
 2,4,6-trimethylpyridine (in MeCN) | MeCN | N Parameter: 9.39 sN Parameter: 0.60 | Synthesis 2017, 49, 3495-3504 DOI: 10.1055/s-0036-1590504 | |
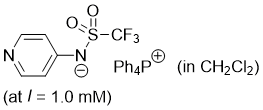 pyridin-4-yl((trifluoromethyl)sulfonyl)amide (Ph4P+) (in CH2Cl2) | dichloromethane | N Parameter: 17.88 sN Parameter: 0.73 | J. Am. Chem. Soc. 2025, 147, 5043-5050 DOI: 10.1021/jacs.4c14825 | |
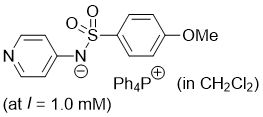 ((4-methoxyphenyl)sulfonyl)(pyridin-4-yl)amide (Ph4P+) (in CH2Cl2) | dichloromethane | N Parameter: 19.63 sN Parameter: 0.65 | J. Am. Chem. Soc. 2025, 147, 5043-5050 DOI: 10.1021/jacs.4c14825 | |
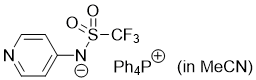 pyridin-4-yl((trifluoromethyl)sulfonyl)amide (Ph4P+) (in MeCN) | MeCN | N Parameter: 16.38 sN Parameter: 0.60 | J. Am. Chem. Soc. 2025, 147, 5043-5050 DOI: 10.1021/jacs.4c14825 | |
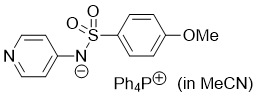 ((4-methoxyphenyl)sulfonyl)(pyridin-4-yl)amide (Ph4P+) (in MeCN) | MeCN | N Parameter: 17.28 sN Parameter: 0.65 | J. Am. Chem. Soc. 2025, 147, 5043-5050 DOI: 10.1021/jacs.4c14825 | |
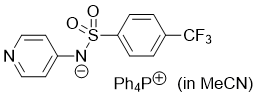 pyridin-4-yl((4-(trifluoromethyl)phenyl)sulfonyl)amide (Ph4P+) (in MeCN) | MeCN | N Parameter: 19.51 sN Parameter: 0.47 | J. Org. Chem. 2025, 90, 2298-2306 DOI: 10.1021/acs.joc.4c02668 | |
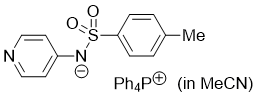 pyridin-4-yl(tosyl)amide (Ph4P+) (in MeCN) | MeCN | N Parameter: 17.19 sN Parameter: 0.64 | J. Org. Chem. 2025, 90, 2298-2306 DOI: 10.1021/acs.joc.4c02668 | |
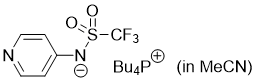 pyridin-4-yl((trifluoromethyl)sulfonyl)amide (Bu4P+) (in MeCN) | MeCN | N Parameter: 16.48 sN Parameter: 0.60 | J. Org. Chem. 2025, 90, 2298-2306 DOI: 10.1021/acs.joc.4c02668 | |
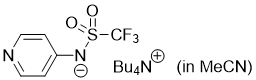 pyridin-4-yl((trifluoromethyl)sulfonyl)amide (Bu4N+) (in MeCN) | MeCN | N Parameter: 16.68 sN Parameter: 0.59 | J. Org. Chem. 2025, 90, 2298-2306 DOI: 10.1021/acs.joc.4c02668 | |
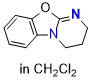 O-DHPB (in CH2Cl2) | dichloromethane | N Parameter: 13.31 sN Parameter: 0.69 | Angew. Chem. Int. Ed. 2025, EarlyView, e202514865 DOI: 10.1002/anie.202514865 | |
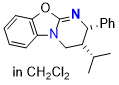 O-HyperBTM (in CH2Cl2) | dichloromethane | N Parameter: 13.01 sN Parameter: 0.73 | Angew. Chem. Int. Ed. 2025, EarlyView, e202514865 DOI: 10.1002/anie.202514865 | |
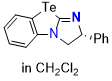 Te-BTM (in CH2Cl2) | dichloromethane | N Parameter: 14.62 sN Parameter: 0.66 | Angew. Chem. Int. Ed. 2025, EarlyView, e202514865 DOI: 10.1002/anie.202514865 | |
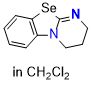 Se-DHPB (in CH2Cl2) | dichloromethane | N Parameter: 15.16 sN Parameter: 0.66 | Angew. Chem. Int. Ed. 2025, EarlyView, e202514865 DOI: 10.1002/anie.202514865 | |
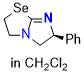 Se-tetramisole (Se-TM) (in CH2Cl2) | dichloromethane | N Parameter: 15.26 sN Parameter: 0.60 | Angew. Chem. Int. Ed. 2025, EarlyView, e202514865 DOI: 10.1002/anie.202514865 | |
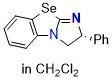 Se-BTM (in CH2Cl2) | dichloromethane | N Parameter: 14.27 sN Parameter: 0.63 | Angew. Chem. Int. Ed. 2025, EarlyView, e202514865 DOI: 10.1002/anie.202514865 | |
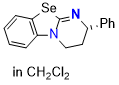 Se-HBTM (in CH2Cl2) | dichloromethane | N Parameter: 14.42 sN Parameter: 0.67 | Angew. Chem. Int. Ed. 2025, EarlyView, e202514865 DOI: 10.1002/anie.202514865 | |
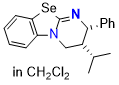 Se-HyperBTM (in DCM) | dichloromethane | N Parameter: 16.11 sN Parameter: 0.58 | Angew. Chem. Int. Ed. 2025, EarlyView, e202514865 DOI: 10.1002/anie.202514865 | |
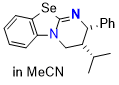 Se-HyperBTM (in MeCN) | MeCN | N Parameter: 15.84 sN Parameter: 0.57 | Angew. Chem. Int. Ed. 2025, EarlyView, e202514865 DOI: 10.1002/anie.202514865 | |
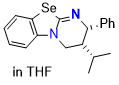 Se-HyperBTM (in THF) | THF | N Parameter: 14.19 sN Parameter: 0.77 | Angew. Chem. Int. Ed. 2025, EarlyView, e202514865 DOI: 10.1002/anie.202514865 | |
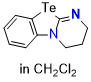 Te-DHPB (in CH2Cl2) | dichloromethane | N Parameter: 16.63 sN Parameter: 0.65 | Angew. Chem. Int. Ed. 2025, EarlyView, e202514865 DOI: 10.1002/anie.202514865 | |
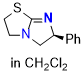 tetramisole (TM) (in CH2Cl2) | dichloromethane | N Parameter: 13.86 sN Parameter: 0.68 | Angew. Chem. Int. Ed. 2025, EarlyView, e202514865 DOI: 10.1002/anie.202514865 | |
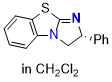 BTM (in CH2Cl2) | dichloromethane | N Parameter: 13.06 sN Parameter: 0.73 | Angew. Chem. Int. Ed. 2025, EarlyView, e202514865 DOI: 10.1002/anie.202514865 | |
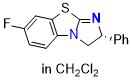 7-F-BTM (in CH2Cl2) | dichloromethane | N Parameter: 13.46 sN Parameter: 0.66 | Angew. Chem. Int. Ed. 2025, EarlyView, e202514865 DOI: 10.1002/anie.202514865 | |
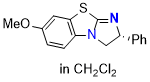 7-MeO-BTM (in CH2Cl29 | dichloromethane | N Parameter: 13.15 sN Parameter: 0.75 | Angew. Chem. Int. Ed. 2025, EarlyView, e202514865 DOI: 10.1002/anie.202514865 |