Pyridines, Quinolines etc.
For reactivities of structurally related compounds go to: Nucleophiles » N-Nucleophiles
Found 56 Molecules.
| Molecule | Solvent | Reactivity Parameters | Classification | Reference (sort by title or year) |
|---|---|---|---|---|
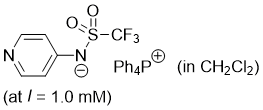 pyridin-4-yl((trifluoromethyl)sulfonyl)amide (Ph4P+) (in CH2Cl2) | dichloromethane | N Parameter: 17.88 sN Parameter: 0.73 | J. Am. Chem. Soc. 2025, 147, 5043-5050 DOI: 10.1021/jacs.4c14825 | |
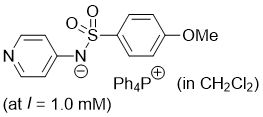 ((4-methoxyphenyl)sulfonyl)(pyridin-4-yl)amide (Ph4P+) (in CH2Cl2) | dichloromethane | N Parameter: 19.63 sN Parameter: 0.65 | J. Am. Chem. Soc. 2025, 147, 5043-5050 DOI: 10.1021/jacs.4c14825 | |
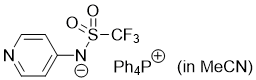 pyridin-4-yl((trifluoromethyl)sulfonyl)amide (Ph4P+) (in MeCN) | MeCN | N Parameter: 16.38 sN Parameter: 0.60 | J. Am. Chem. Soc. 2025, 147, 5043-5050 DOI: 10.1021/jacs.4c14825 | |
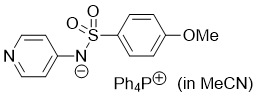 ((4-methoxyphenyl)sulfonyl)(pyridin-4-yl)amide (Ph4P+) (in MeCN) | MeCN | N Parameter: 17.28 sN Parameter: 0.65 | J. Am. Chem. Soc. 2025, 147, 5043-5050 DOI: 10.1021/jacs.4c14825 | |
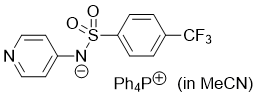 pyridin-4-yl((4-(trifluoromethyl)phenyl)sulfonyl)amide (Ph4P+) (in MeCN) | MeCN | N Parameter: 19.51 sN Parameter: 0.47 | J. Org. Chem. 2025, 90, 2298-2306 DOI: 10.1021/acs.joc.4c02668 | |
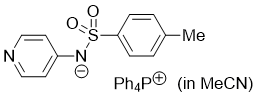 pyridin-4-yl(tosyl)amide (Ph4P+) (in MeCN) | MeCN | N Parameter: 17.19 sN Parameter: 0.64 | J. Org. Chem. 2025, 90, 2298-2306 DOI: 10.1021/acs.joc.4c02668 | |
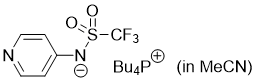 pyridin-4-yl((trifluoromethyl)sulfonyl)amide (Bu4P+) (in MeCN) | MeCN | N Parameter: 16.48 sN Parameter: 0.60 | J. Org. Chem. 2025, 90, 2298-2306 DOI: 10.1021/acs.joc.4c02668 | |
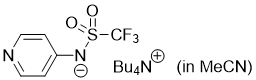 pyridin-4-yl((trifluoromethyl)sulfonyl)amide (Bu4N+) (in MeCN) | MeCN | N Parameter: 16.68 sN Parameter: 0.59 | J. Org. Chem. 2025, 90, 2298-2306 DOI: 10.1021/acs.joc.4c02668 | |
 pyridine (in MeCN) | MeCN | N Parameter: 13.60 sN Parameter: 0.60 | Synthesis 2017, 49, 3495-3504 DOI: 10.1055/s-0036-1590504 | |
 2-methylpyridine (in CH2Cl2) | dichloromethane | N Parameter: 10.52 sN Parameter: 0.78 | Synthesis 2017, 49, 3495-3504 DOI: 10.1055/s-0036-1590504 | |
 2-methylpyridine (in MeCN) | MeCN | N Parameter: 10.98 sN Parameter: 0.66 | Synthesis 2017, 49, 3495-3504 DOI: 10.1055/s-0036-1590504 | |
 2,6-dimethylpyridine (in CH2Cl2) | dichloromethane | N Parameter: 9.87 sN Parameter: 0.68 | Synthesis 2017, 49, 3495-3504 DOI: 10.1055/s-0036-1590504 | |
 2,6-dimethylpyridine (in MeCN) | MeCN | N Parameter: 9.11 sN Parameter: 0.69 | Synthesis 2017, 49, 3495-3504 DOI: 10.1055/s-0036-1590504 | |
 2,4,6-trimethylpyridine (in CH2Cl2) | dichloromethane | N Parameter: 9.34 sN Parameter: 0.71 | Synthesis 2017, 49, 3495-3504 DOI: 10.1055/s-0036-1590504 | |
 2,4,6-trimethylpyridine (in MeCN) | MeCN | N Parameter: 9.39 sN Parameter: 0.60 | Synthesis 2017, 49, 3495-3504 DOI: 10.1055/s-0036-1590504 | |
 nicotine (in CH2Cl2) | dichloromethane | N Parameter: 10.40 sN Parameter: 1.04 | J. Phys. Org. Chem. 2016, 29, 759-767 DOI: 10.1002/poc.3580 | |
 nicotine (in MeCN) | MeCN | N Parameter: 11.60 sN Parameter: 0.81 | J. Phys. Org. Chem. 2016, 29, 759-767 DOI: 10.1002/poc.3580 | |
 3-methylpyridine (in CH2Cl2) | dichloromethane | N Parameter: 10.90 sN Parameter: 0.93 | J. Phys. Org. Chem. 2016, 29, 759-767 DOI: 10.1002/poc.3580 | |
 3-methylpyridine (in MeCN) | MeCN | N Parameter: 11.50 sN Parameter: 0.80 | J. Phys. Org. Chem. 2016, 29, 759-767 DOI: 10.1002/poc.3580 | |
 4-(morpholino)pyridine (in CH2Cl2) | dichloromethane | N Parameter: 14.59 sN Parameter: 0.69 | Eur. J. Org. Chem. 2016, , 4050-4058 DOI: 10.1002/ejoc.201600572 | |
 4-(2-(fluorodiphenylmethyl)pyrrolidin-1-yl)pyridine | dichloromethane | N Parameter: 14.57 sN Parameter: 0.75 | Eur. J. Org. Chem. 2014, , 1202-1211 DOI: 10.1002/ejoc.201301730 | |
 TCAP (in MeCN) | MeCN | N Parameter: 15.60 sN Parameter: 0.68 | Chem. Eur. J. 2013, 19, 6435-6442 DOI: 10.1002/chem.201204452 | |
 4-pyrrolidinopyridine (in MeCN) | MeCN | N Parameter: 14.99 sN Parameter: 0.69 | Chem. Eur. J. 2013, 19, 6435-6442 DOI: 10.1002/chem.201204452 | |
 4-(dimethylamino)pyridine (in MeCN) | MeCN | N Parameter: 15.51 sN Parameter: 0.62 | J. Phys. Chem. A 2012, 116, 8494-8499 DOI: 10.1021/jp3049247 | |
 4-(morpholino)pyridine (in MeCN) | MeCN | N Parameter: 14.80 sN Parameter: 0.63 | J. Phys. Chem. A 2012, 116, 8494-8499 DOI: 10.1021/jp3049247 | |
 3-bromo-4-(dimethylamino)pyridine (in MeCN) | MeCN | N Parameter: 12.96 sN Parameter: 0.67 | J. Phys. Chem. A 2012, 116, 8494-8499 DOI: 10.1021/jp3049247 | |
 4-amino-3,5-dibromo-pyridine (in MeCN) | MeCN | N Parameter: 11.11 sN Parameter: 0.75 | J. Phys. Chem. A 2012, 116, 8494-8499 DOI: 10.1021/jp3049247 | |
 4-acetamido-pyridine (in MeCN) | MeCN | N Parameter: 13.24 sN Parameter: 0.67 | J. Phys. Chem. A 2012, 116, 8494-8499 DOI: 10.1021/jp3049247 | |
 2-Me super-dmap (in MeCN) | MeCN | N Parameter: 16.65 sN Parameter: 0.58 | Org. Lett. 2011, 13, 530-533 DOI: 10.1021/ol1029589 | |
 2-Et super-dmap (in MeCN) | MeCN | N Parameter: 16.81 sN Parameter: 0.60 | Org. Lett. 2011, 13, 530-533 DOI: 10.1021/ol1029589 | |
 2-Bn super-dmap (in MeCN) | MeCN | N Parameter: 17.69 sN Parameter: 0.57 | Org. Lett. 2011, 13, 530-533 DOI: 10.1021/ol1029589 | |
 2-Ac super-dmap (in MeCN) | MeCN | N Parameter: 15.39 sN Parameter: 0.60 | Org. Lett. 2011, 13, 530-533 DOI: 10.1021/ol1029589 | |
 2-Bz super-dmap (in MeCN) | MeCN | N Parameter: 14.19 sN Parameter: 0.67 | Org. Lett. 2011, 13, 530-533 DOI: 10.1021/ol1029589 | |
 4-(dimethylamino)pyridine (in THF) | THF | N Parameter: 15.90 sN Parameter: 0.66 | Angew. Chem. Int. Ed. 2011, 50, 6915-6919 DOI: 10.1002/anie.201102435 | |
 6-methoxy-quinoline (in MeCN) | MeCN | N Parameter: 10.86 sN Parameter: 0.66 | J. Org. Chem. 2009, 74, 7157-7164 DOI: 10.1021/jo901670w | |
 4-methyl-quinoline alias lepidine (in MeCN) | MeCN | N Parameter: 11.60 sN Parameter: 0.62 | J. Org. Chem. 2009, 74, 7157-7164 DOI: 10.1021/jo901670w | |
 2-(naphthylmethyl)quinuclidine (in MeCN) | MeCN | N Parameter: 15.66 sN Parameter: 0.62 | J. Org. Chem. 2009, 74, 7157-7164 DOI: 10.1021/jo901670w | |
 quinine | dichloromethane | N Parameter: 10.46 sN Parameter: 0.75 | J. Org. Chem. 2009, 74, 7157-7164 DOI: 10.1021/jo901670w | |
 quinidine | dichloromethane | N Parameter: 10.54 sN Parameter: 0.74 | J. Org. Chem. 2009, 74, 7157-7164 DOI: 10.1021/jo901670w | |
 4-chloropyridine (in CH2Cl2) | dichloromethane | N Parameter: 11.70 sN Parameter: 0.67 | Chem. Eur. J. 2007, 13, 336-345 DOI: 10.1002/chem.200600941 | |
 4-chloropyridine (in H2O) | water | N Parameter: 10.50 sN Parameter: 0.73 | Chem. Eur. J. 2007, 13, 336-345 DOI: 10.1002/chem.200600941 | |
 pyridine (in CH2Cl2) | dichloromethane | N Parameter: 12.90 sN Parameter: 0.67 | Chem. Eur. J. 2007, 13, 336-345 DOI: 10.1002/chem.200600941 | |
 pyridine (in H2O) | water | N Parameter: 11.05 sN Parameter: 0.73 | Chem. Eur. J. 2007, 13, 336-345 DOI: 10.1002/chem.200600941 | |
 4-methylpyridine (in CH2Cl2) | dichloromethane | N Parameter: 13.70 sN Parameter: 0.67 | Chem. Eur. J. 2007, 13, 336-345 DOI: 10.1002/chem.200600941 | |
 4-methylpyridine (in H2O) | water | N Parameter: 11.10 sN Parameter: 0.75 | Chem. Eur. J. 2007, 13, 336-345 DOI: 10.1002/chem.200600941 | |
 4-methoxypyridine (in CH2Cl2) | dichloromethane | N Parameter: 13.70 sN Parameter: 0.67 | Chem. Eur. J. 2007, 13, 336-345 DOI: 10.1002/chem.200600941 | |
 4-methoxypyridine (in H2O) | water | N Parameter: 11.44 sN Parameter: 0.68 | Chem. Eur. J. 2007, 13, 336-345 DOI: 10.1002/chem.200600941 | |
 4-aminopyridine (in CH2Cl2) | dichloromethane | N Parameter: 15.20 sN Parameter: 0.67 | Chem. Eur. J. 2007, 13, 336-345 DOI: 10.1002/chem.200600941 | |
 4-aminopyridine (in H2O) | water | N Parameter: 12.19 sN Parameter: 0.66 | Chem. Eur. J. 2007, 13, 336-345 DOI: 10.1002/chem.200600941 | |
 4-(dimethylamino)pyridine (in CH2Cl2) | dichloromethane | N Parameter: 15.80 sN Parameter: 0.66 | Chem. Eur. J. 2007, 13, 336-345 DOI: 10.1002/chem.200600941 | |
 4-(dimethylamino)pyridine (in H2O) | water | N Parameter: 13.19 sN Parameter: 0.56 | Chem. Eur. J. 2007, 13, 336-345 DOI: 10.1002/chem.200600941 | |
 4-(dimethylamino)pyridine (in 91M9AN) | MeOH-MeCN mix | N Parameter: 13.20 sN Parameter: 0.67 | Chem. Eur. J. 2007, 13, 336-345 DOI: 10.1002/chem.200600941 | |
 4-(dimethylamino)pyridine (in DMSO) | DMSO | N Parameter: 14.80 sN Parameter: 0.67 | Chem. Eur. J. 2007, 13, 336-345 DOI: 10.1002/chem.200600941 | |
 4-(dimethylamino)pyridine (in DMF) | DMF | N Parameter: 14.90 sN Parameter: 0.67 | Chem. Eur. J. 2007, 13, 336-345 DOI: 10.1002/chem.200600941 | |
 4-pyrrolidinopyridine (in CH2Cl2) | dichloromethane | N Parameter: 15.90 sN Parameter: 0.67 | Chem. Eur. J. 2007, 13, 336-345 DOI: 10.1002/chem.200600941 | |
 4-pyrrolidinopyridine (in H2O) | water | N Parameter: 12.39 sN Parameter: 0.66 | Chem. Eur. J. 2007, 13, 336-345 DOI: 10.1002/chem.200600941 |